Abstract
We examined the clinical and laboratory characteristics of children newly diagnosed with diabetes mellitus (DM) in a single-center study. We retrospectively reviewed the data of 155 children with DM between January 2000 and December 2013. Of 155 diabetic children, 87 (56.1%) were diagnosed with type 1 DM (T1DM) and 68 (43.9%) with type 2 DM (T2DM). Mean ages at diagnosis were 8.95±3.89 years (T1DM) and 13.76±2.23 years (T2DM), respectively (p<0.001). There were significant differences in HbA1c, C-peptide, and glutamic acid decarboxylase antibody levels between the T1DM and T2DM groups. Annual numbers of children with DM have increased, and since 2011 the number of children with T2DM has surpassed the number with T1DM. The most common clinical symptom in T1DM was polyuria, and 26.4% of children with T1DM presented initially with diabetic ketoacidosis. In contrast, 60.3% of T2DM children showed glucosuria in a school urine screening, and only 19.1% presented with polydipsia. The rate of positivity for at least more than one islet autoantibody was 77.1% in T1DM and 26.3% in T2DM. Serum C-peptide levels in T2DM were increased up to 12 months after onset and remained >3.59 ng/mL for 36 months. However, serum C-peptide levels in T1DM were slightly increased up to 6 months after onset and gradually decreased to 0.32 ng/mL for 36 months. The prevalence of children with DM has increased over the last 14 years, and the proportion of T2DM patients has rapidly increased since 2009. Because childhood DM is associated with several metabolic and cardiovascular complications, children should be screened for early detection of DM, especially asymptomatic T2DM in children and adolescents.
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin resistance of action, or both. DM can usually be diagnosed by serum glucose concentrations and the diagnosis is easily confirmed. Insulin and C-peptide concentrations reflect the function of pancreatic cells and are an important diagnostic tool. Absolute deficiency of insulin most commonly results from an autoimmune destruction of cells in the pancreas and is referred to as type 1 DM (T1DM).1 In most Western countries, T1DM makes up over 90% of childhood DM, although less than half of patients with DM are detected before the age of 15 years.23 The incidence of DM is increasing worldwide, and recent studies have shown a gradual and steady increase in the incidence of both T1DM and type 2 diabetes mellitus (T2DM).4 DM affects approximately 1 in 500 young people, with 85% of patients diagnosed as having T1DM, 12% as having T2DM, and 3% as having other types. However, T2DM is becoming more common and accounts for a significant proportion of childhood DM in some at-risk populations, with a similarity to the proportions of childhood obesity.56 In most patients with DM, classification can reliably be made on the basis of clinical presentation, clinical course, and laboratory findings. However, T1DM and T2DM are not completely distinct, with considerable overlap, and some patients may have mixed features. Therefore, children and adolescents with diabetes should be diagnosed for classification of T1DM and T2DM for adequate serum glucose control.
The aim of this study was to examine the type, prevalence, and clinical and laboratory characteristics of children and adolescents newly diagnosed with DM between January 2000 and December 2013 in a single center.
This study included 155 children and adolescents with DM. All subjects had been newly diagnosed with DM between January 2000 and December 2013 and received follow-up care at the Pediatric Endocrinology Department of Chonbuk National University Children's Hospital. All subjects were categorized into T1DM and T2DM groups and the age distribution of the subjects was between 1 and 18 years old. Exclusion criteria were (1) previously diagnosed DM, (2) cases transferred from other hospitals after diagnosis, (3) highly suspected maturity-onset diabetes of the young (MODY) on the basis of family history, and (4) age less than 3 months. The Institutional Review Board of the Clinical Research Institute in Chonbuk National University Children's Hospital approved the study protocol.
We reviewed the medical records of the 155 patients and obtained the patients' clinical characteristics and laboratory data retrospectively. Laboratory findings at the time of diagnosis included fasting serum glucose, C-peptide, hemoglobin A1c (HbA1c), islet autoantibodies [glutamic acid decarboxylase antibody (GADA) and insulin autoantibody (IAA)], thyroid autoantibodies, and HLA typing. We assessed markers of pancreatic autoimmunity and considered GADA ≥0.9 units/mL or IAA level ≥7% as positive findings. GADA values were measured by immunoradiometric assay by use of a GADA assay kit (RSR Ltd., Cardiff, UK). Concentrations of IAA were measured by radioimmunoassay with the AIA-100 kit (Biosource, Nivelles, Belgium), respectively.
Diagnostic criteria for diabetes mellitus were based on clinical and laboratory findings and the WHO classification of diabetes. The diagnosis of DM was based on (1) symptoms of diabetes plus random plasma glucose level ≥200 mg/dL, (2) fasting plasma glucose level ≥126 mg/dL, or (3) 2-hour post-load glucose level ≥200 mg/dL. The classification of DM was based on clinical and laboratory characteristics including fasting serum C-peptide, pancreatic islet cell autoantibodies, HLA type, clinical course, and symptoms. T1DM accounts for pancreatic cell destruction. The diagnostic criteria for T1DM included (1) newly diagnosed T1DM with diabetic ketoacidosis, (2) positive for both pancreatic autoantibodies, (3) a serum C-peptide level below 0.6 ng/mL, and (4) if a serum C-peptide level above 0.6 ng/mL, a requirement for insulin therapy to maintain blood glucose levels.
Statistical analysis was performed by using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The independent two-sample t-test was used to compare the levels of serum C-peptide, age, HbA1c, GADA, and IAA levels between the T1DM and T2DM groups. The results were expressed as means±standard deviations. Statistical significance was defined as p<0.05 for all clinical and laboratory data.
The characteristics of the 155 children and adolescents with newly diagnosed DM are shown in Table 1. Children and adolescents diagnosed with T1DM accounted for 87 of the 155 patients (56.1%); the remaining 68 patients (43.9%) were diagnosed with T2DM. Of the children and adolescents with T1DM, 36 were male and 51 were female; of the children with T2DM, 32 were male and 36 were female. Children and adolescents with T1DM were less likely to have a family history of T2DM (39.0% vs. 57.4%, respectively, p<0.001) and had lower C-peptide levels (0.92±0.70 vs. 3.77±2.15 ng/mL, respectively, p<0.001) but higher serum HbA1c levels (11.88±2.39% vs. 10.07±2.67%, respectively, p<0.001) than did children with T2DM. For the pancreatic autoantibodies, GADA and IAA levels were significantly higher in children with T1DM than in those with T2DM (21.59±31.23 vs. 1.49±9.73 unit/mL, p<0.001, and 9.21±9.90 vs. 5.17±2.44, p=0.01, respectively).
The mean annual number of subjects with DM was 11.1 (5 to 27 cases per year). The proportion of children and adolescents with new-onset DM gradually increased over the last 14 years (Fig. 1). The proportion of subjects with T1DM was higher than that of subjects with T2DM until 2010. However, the number of subjects with T2DM has increased remarkably since 2007, and the total annual number of subjects with T2DM has surpassed that of T1DM since 2011. The mean age of all subjects with DM was 11.1 years, and subjects with T1DM had a significantly earlier age at onset than did children with T2DM (8.95±3.89 vs 13.76±2.23 years, p<0.01, Fig. 2A). More than 50% of subjects were diagnosed with DM between the ages of 10 and 15 years (Fig. 2B). Among 87 subjects with T1DM, only 4 (4.6%) were diagnosed over the age of 15 years. In addition, no subject with T2DM was younger than 8 years (Fig. 2B).
As shown in Fig. 3, clinical symptoms differed significantly between patients with T1DM and T2DM. Common clinical symptoms in T1DM included polyuria, polydipsia, and weight loss; 26.4% of children presented initially with diabetic ketoacidosis (DKA). In contrast, 60.3% of T2DM subjects showed only glucosuria on a urine screening test, and less than 22.0% of T2DM subjects presented with polydipsia. Obesity was present in 49.1% of patients with T2DM. In addition, no subjects with T2DM presented with DKA at the time of diagnosis.
In this study, 140 subjects with DM were tested for the pancreatic autoantibodies GADA and IAA. As shown in Fig. 4, there was a significant difference in positivity for pancreatic autoantibodies between patients with T1DM and T2DM. Seventy-nine of 140 subjects (56.4%) were positive for at least more than one autoantibody (Fig. 4; T1DM, 77.1%; T2DM, 26.3%). Twenty-seven subjects (19.3%) were positive for both GADA and IAA autoantibodies, and they were all diagnosed with T1DM. Of 52 subjects who were positive for either GADA or IAA, 71.2% subjects were diagnosed with T1DM. In addition, 61 subjects (43.6%) were negative for both GADA and IAA autoantibodies, and 68.9% of them were T2DM subjects.
We serially examined serum C-peptide levels at 6-month intervals for 3 years after diagnosis in 21 subjects with DM (T1DM=14; T2DM=7). Initial C-peptide levels were significantly lower in patients with T1DM than in those with T2DM (0.97±0.9 vs 2.91±1.88 ng/mL, respectively, p<0.05). During the follow-up period, serum C-peptide levels in T2DM increased up to 12 months after onset and remained >3.59 ng/mL for the following 36 months (Fig. 5). However, serum C-peptide levels in patients with T1DM were slightly increased up to 6 months after onset and gradually decreased to the level of 0.32 ng/mL by 36 months after diagnosis.
DM is a chronic disorder related to pancreatic islet cells, especially insulin and C-peptide. Classification of DM is dependent on insulin function, and the type of DM can sometimes change. T1DM, the most prominent form of DM seen in children and adolescents, is an autoimmune disease characterized by destruction of the insulin-producing β-cells in the pancreas, leading to total or near total insulin deficiency.1 T2DM is characterized by chronic hyperglycemia and insulin resistance that causes a relative insulin deficiency.7 In T2DM, the defining factor is insulin resistance, and contributors to insulin resistance include genetic factors, obesity, family history, reduced physical activity, high or low birth weight, and infection.8 However, it can be difficult to classify DM in some cases, because of overlap in the presentation of typical clinical symptoms between T1DM and T2DM. For instance, pancreatic autoantibodies can be detected in some children and adolescents with T2DM, and widespread and rapid increases in childhood obesity mean that even patients with T1DM present with obesity.49
The incidence of DM is increasing worldwide.4 In most Western countries, T1DM accounts for over 90% of childhood and adolescent DM, although less than half of patients with T1DM are detected before the age of 15 years.9 T1DM incidence varies between countries, within countries, and between ethnic populations.1011 Childhood diabetes mellitus is thought of as T1DM, but the incidence of T2DM in childhood is steady increasing. T2DM, previously considered a condition that primarily affects the middle aged or elderly, is increasingly being reported among children and adolescents.4 This is likely owing to the emerging epidemic of youth obesity and decreased physical activity owing to changes in social play.2 Several studies have shown an increased risk of microvascular complications in youth with T2DM compared with those with T1DM.56 Thus, the social and economic effects may be increasing. In our study, the numbers of children and adolescents with DM, including T1DM, increased annually during the last 14 years. Although T1DM is still the major form of DM in children and adolescents, we observed increases in the number of subjects with T2DM since 2007, and recently, the total annual number of subjects with T2DM surpassed that of subjects with T1DM. Although children with T1DM had a significantly earlier age of onset than did those with T2DM, more than 50% of all subjects were diagnosed between the ages of 10 and 15 years. We also found that no subjects with T2DM were diagnosed under the age of 8 years.
In this study, children and adolescents with T2DM had significantly fewer clinical symptoms than did patients with T1DM. Polyuria was the most common symptom in subjects with T1DM, and 26.4% of subjects showed DKA. DKA is the leading cause of mortality and morbidity in children with T1DM.4 DKA in children develops quickly and is much more prevalent than in adults. There is wide geographic variation in the frequency of DKA at DM onset.4 The presence of DKA at initial presentation in our study was comparatively higher than that reported in Sweden (12.8%) and Finland (19%).1213 In contrast, we found that less than 22.0% of subjects with T2DM presented with polydipsia and polyuria, and none presented with DKA. Furthermore, 60.3% of T2DM subjects showed only asymptomatic glucosuria on urine screening. In our study, 41 of 68 subjects with T2DM were detected by a urine glucose screening. They all had asymptomatic glucosuria and were diagnosed as having DM by oral glucose tolerance test. The main purpose of the urine glucose screening was to identify children and adolescents with DM, especially T2DM, in the early stages of the condition.7 According to Japanese urine glucose screening studies,10 the incidence of T2DM in children has increased over the last three decades, and was estimated to be 3.0 per 100,000 children from 1975 to 2000. Especially, the incidence of junior high school patients as screened by a urine test was growing. Those authors also observed that most cases of DM detected by the screening program were eventually diagnosed as having T2DM and that more than 80% of the children with T2DM were obese.101113 In Korea, more than 4 million schoolchildren have participated in the annual urine screening program since 1998, and approximately 3200 students per year are found to have glucosuria.14 Therefore, the prevalence of diabetes mellitus as detected by urine screening is growing. In our study, most T2DM patients were detected by the screening program at school, and 49.1% of the subjects with T2DM were obese.
The presence of autoantibodies is a sensitive marker for classification and treatment of DM. Compared with that in Western countries, the positivity for pancreatic autoantibodies is relatively lower in Korean children (50%-60% vs. 70-90%).151617 In addition, some studies reported that nearly 30% of European children and adolescents clinically appearing as having T2DM were positive for pancreatic autoantibodies.18 In a previous study, Kong et al reported that the overall prevalence of islet autoantibodies (GADA, ICA512, or IAA) was 71.43% in children and adults with T1DM.19 In this study, we examined GADA and IAA pancreatic autoantibodies. About 77% of subjects with T1DM were positive for at least more than one autoantibody, and 27 subjects who had both GADA and IAA autoantibodies were diagnosed with T1DM. However, only 19.3% of T2DM subjects had GADA or IAA. In cases with several autoantibodies, patients need higher therapeutic doses of insulin and their pancreatic β-cell function rapidly decreases. There were no cases of both GADA and IAA positivity in T2DM.
The serum C-peptide level reflects insulin secretion from pancreatic islet cells and residual insulin secretion function and can be used to differentiate diabetes from other diseases. C-peptide is a useful parameter in determining the correct type of DM. Katz et al identified that a fasting C-peptide concentration >0.3 nmol/L had 83% sensitivity and 89% specificity for distinguishing pediatric T1DM from T2DM at diagnosis.20 In a previous study, we found that if the serum C-peptide level was <0.6 ng/mL at diagnosis, T2DM could be excluded.21 As expected in this study, serum C-peptide levels were significantly lower in patients with T1DM (0.92±0.7 ng/mL) than in those with T2DM (3.77±2.15 ng/mL). The abrupt increase in the C-peptide level in T2DM during the first 1 year of follow-up may have been the result of selection bias because one of the patient's C-peptide levels was very high. In childhood-onset T1DM, the majority of patients are severely insulin deficient within 2 to 3 years of diagnosis, whereas in T2DM, C-peptide persists.22 To evaluate residual β-cell function, we examined serum C-peptide levels at 6-month intervals for 3 years in 21 subjects with DM. In general, the serum C-peptide level of absolute insulin deficiency or absolute insulin requirement was <0.08 nmol/L (0.24 ng/mL) in the fasting state.23 Although serum C-peptide levels were low at diagnosis in T1DM subjects, levels at 36 months were 0.32 ng/mL, suggesting residual insulin secretion function. This finding suggests that intensive insulin therapy is needed to preserve remaining β-cell function in patients with T1DM. In contrast, serum C-peptide levels in patients with T2DM were increased up to 12 months after diagnosis and remained >3.59 ng/mL for 36 months of follow-up.
This study had some limitations. Foremost, it was a retrospective study based on medical chart records, which can cause errors. Second, we excluded subjects with highly suspected MODY on the basis of family history, and some of these subjects could have been T2DM patients. Third, because this was a single-center study, the number of participants was relatively small. Therefore, multicenter studies with a larger number of subjects are needed to understand the epidemiologic changes in prevalence and type of childhood- and adolescent-onset DM in Korea.
In conclusion, the annual number of children with T1DM and T2DM has increased constantly and the proportion of T2DM cases has increased rapidly since 2007. The rising incidence of T2DM mirrors the epidemic of childhood obesity and physical inactivity. Although the majority of T1DM subjects presented with polydipsia, polyuria, and DKA, the majority of patients with T2DM had only glucosuria without other diabetic symptoms. DM in children and adolescents is associated with several metabolic and cardiovascular complications in view of social and economic costs; therefore, children should be screened for early detection of DM, especially asymptomatic T2DM.
Figures and Tables
FIG. 1
Annual distributions of subjects with T1DM and T2DM from 2000 to 2013. T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus.

FIG. 2
Distributions of subjects with T1DM and T2DM according to (A) age at diagnosis and (B) ranges of age. T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus.

FIG. 3
Clinical symptoms of subjects with T1DM and T2DM at diagnosis. T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus, DKA: diabetic ketoacidosis.

FIG. 4
Positivity for pancreatic autoantibodies in subjects with T1DM and T2DM. T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus, HbA1c: hemoglobin A1c, GADA: glutamic acid decarboxylase antibody, IAA: insulin autoantibody.
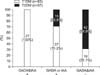
FIG. 5
Mean serum C-peptide levels during first 3 years of follow-up in subjects with T1DM (n=14) and T2DM (n=7). T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus.

References
1. Dejkhamron P, Menon RK, Sperling MA. Childhood diabetes mellitus: recent advances & future prospects. Indian J Med Res. 2007; 125:231–250.
2. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997; 20:1183–1197.
3. Sperling MA. Diabetes Mellitus. In : Sperling MA, editor. Pediatric endocrinology. 2nd ed. Philadelphia: Saunders;2002. p. 323–366.
4. Aanstoot HJ, Anderson BJ, Daneman D, Danne T, Donaghue K, Kaufman F, et al. The global burden of youth diabetes: perspectives and potential. Pediatr Diabetes. 2007; 8:Suppl 8. 1–44.
6. Ize-Ludlow D, Sperling MA. The classification of diabetes mellitus: a conceptual framework. Pediatr Clin North Am. 2005; 52:1533–1552.

7. Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013; 4:270–281.

8. Arslanian S. Type 2 diabetes in children: clinical aspects and risk factors. Horm Res. 2002; 57:Suppl 1. 19–28.

9. Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009; 10:Suppl 12. 3–12.

10. Barrett TG. Non autoimmune forms of diabetes: Neonatal diabetes, DIDMOAAD-Wolfram, and related syndromes. In : Sperling MA, editor. Type 1 Diabetes. Totowa: Humana Press;2003. p. 163–182.
11. Porter JR, Barrett TG. Acquired non-type 1 diabetes in childhood: subtypes, diagnosis, and management. Arch Dis Child. 2004; 89:1138–1144.

12. Samuelsson U, Stenhammar L. Clinical characteristics at onset of Type 1 diabetes in children diagnosed between 1977 and 2001 in the south-east region of Sweden. Diabetes Res Clin Pract. 2005; 68:49–55.

13. Hekkala A, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes in children in northern Finland: temporal changes over 20 years. Diabetes Care. 2007; 30:861–866.

14. Morimoto A, Nishimura R, Tajima N. Trends in the epidemiology of patients with diabetes in Japan. JMAJ. 2010; 53:36–40.
15. Lee CW, Shin HJ, Kim DH. Prevalence of autoimmune antibodies in type i diabetic children and their siblings. J Korean Soc Pediatr Endocrinol. 1999; 4:78–87.
16. Hong EH, Park JS, Lee HS, Cho MH, Ko CW. Clinical characteristics and laboratory findings of children who were newly diagnosed with diabetes mellitus (From 2001 to 2008). J Korean Soc Pediatr Endocrinol. 2009; 14:110–115.
17. Yu J, Shin CH, Yang SW, Park MH, Eisenbarth GS. Analysis of children with type 1 diabetes in Korea: high prevalence of specific anti-islet autoantibodies, immunogenetic similarities to Western populations with "unique" haplotypes, and lack of discrimination by aspartic acid at position 57 of DQB. Clin Immunol. 2004; 113:318–325.

18. Klingensmith GJ, Pyle L, Arslanian S, Copeland KC, Cuttler L, Kaufman F, et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care. 2010; 33:1970–1975.

19. Kong YH, Kim MS, Lee DY. Comparison of the prevalence of islet autoantibodies according to age and disease duration in patients with type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab. 2013; 18:65–70.

20. Katz LE, Jawad AF, Ganesh J, Abraham M, Murphy K, Lipman TH. Fasting c-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabetes. 2007; 8:53–59.

21. Cho MJ, Kim MS, Kim CJ, Kim EY, Kim JD, Kim EY, et al. Fasting serum C-peptide is useful for initial classification of diabetes mellitus in children and adolescents. Ann Pediatr Endocrinol Metab. 2014; 19:80–85.

22. Besser RE. Determination of C-peptide in children: when is it useful. Pediatr Endocrinol Rev. 2013; 10:494–502.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download