Abstract
Osteoclasts are multinucleated cells of hematopoietic origin that are responsible for the degradation of old bone matrix. Osteoclast differentiation and activity are controlled by two essential cytokines, macrophage colony-stimulating factor (M-CSF) and the receptor activator of nuclear factor-κB ligand (RANKL). M-CSF and RANKL bind to their respective receptors c-Fms and RANK to stimulate osteoclast differentiation through regulation of delicate signaling systems. Here, we summarize the critical or essential signaling pathways for osteoclast differentiation including M-CSF-c-Fms signaling, RANKL-RANK signaling, and costimulatory signaling for RANK.
Bone is actually a dynamic organ.1 Normal bone mass and strength are maintained by constant "bone remodeling" processes.2 The old bone is degraded by osteoclasts and replaced by osteoblasts. Therefore, the balance between osteoblastic bone formation and osteoclastic bone resorption is important for normal bone homeostasis. Excessive bone resorption by osteoclasts often causes osteopenic diseases including osteoporosis and rheumatoid arthritis.1
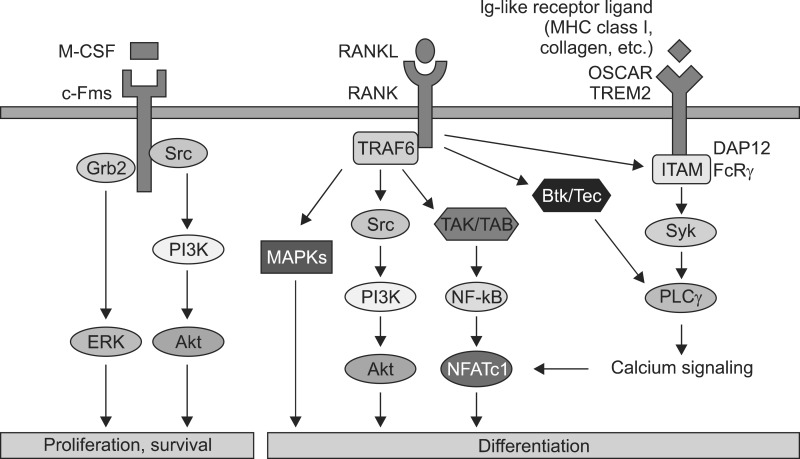
Osteoclasts differentiate from bone marrow monocyte/ macrophage lineage cells under the control of two essential cytokines.3 The binding of the macrophage colony-stimulating factor (M-CSF) to c-Fms provides signals required for proliferation and survival of osteoclast precursor cells, whereas the binding of receptor activator of nuclear factor-κB ligand (RANKL) to RANK stimulates signals required for osteoclast differentiation and the resorptive function as well as the survival of mature osteoclasts.45678 Recently, several genetic studies have shown that additional signals that can support RANK signaling are necessary for the full differentiation of osteoclasts (Fig. 1).910
Here, we discuss the important signaling pathways in osteoclast differentiation.
The pivotal roles of M-CSF (CSF-1) in osteoclast differentiation were revealed by studies using mice (op/op) and rats (tl/tl). The op/op mice and tl/tl rats, which have a point mutation in the csf1 gene and express non-functional M-CSF, develop severe osteopetrotic bone phenotypes due to a complete absence of osteoclasts.1112 M-CSF plays an important role in supporting the proliferation and survival of osteoclast precursor cells. The binding of M-CSF to its cognate receptor c-Fms results in auto-and trans-phosphorylation of specific tyrosine residues in the cytoplasmic tail of c-Fms. It has been well demonstrated that four (underlined) of the eight tyrosine residues (Y544, Y559, Y697, Y706, Y721, Y807, Y921, and Y974) within the cytoplasmic tail of c-Fms functionally regulate the proliferation and survival of osteoclast precursor cells.131415161718 Particularly, phosphorylation of Y559 is required for the full activation of c-Fms. Phosphorylated Y559 interacts with c-Src.19 The resulting phosphor-Y559/c-Src complex recruits the phosphatidylinositol 3-kinase (PI3K) and c-Cbl complex, which in turn activates the Akt pathway and causes c-Fms ubiquitination, respectively.2021 The c-Cbldependent c-Fms ubiquitination augments its tyrosine phosphorylation and activation via a conformational change in the kinase domain. Phosphorylated Y721 also activates the Akt pathway through direct interaction with PI3K.2022 On the other hand, phosphorylated Y697 and Y974 interact with Grb2 to mediate activation of ERK.23 Therefore, M-CSF-induced activation of c-Fms results in enhanced osteoclast precursor proliferation and survival through the ERK and PI3K/Akt pathways. Although binding partners and the precise signaling mechanism have not been fully identified, phosphorylation of Y544 and Y807 are also required for c-Fms activation and osteoclast differentiation.1824 The pivotal roles of M-CSF in osteoclast differentiation are also supported by analysis of the csf1r (gene coding c-Fms)-lacking mice, which exhibit an osteopetrotic bone phenotype.25
RANKL (OPGL, ODF, and TRANCE) and its cognate receptor RANK are also key osteoclastogenic factors.26 Osteopetrotic bone phenotypes without osteoclasts of both RANKL-and RANK-deficient mice have well revealed that both factors are implicated in regulating osteoclast formation and function.2728
Binding of RANKL to RANK leads to recruitment of TNF receptor-associated factor (TRAF) adaptor proteins including TRAFs 1, 2, 3, 5, and 6 to the conserved TRAF domain within the cytoplasmic domain of RANK.2930 Among the TRAF members, TRAF6 is the most critical for osteoclast formation and function since TRAF6-lacking mice develop severe osteopetrosis owing to impaired osteoclast differentiation or bone resorption.3132 TRAF6 transmits the RANKL/RANK signal to downstream targets such as nuclear factor kappa B (NF-κB), c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), p38, Akt, and NFATc1.83132333435 However, IL-1 administration failed to induce osteoclast differentiation in RANK knockout mice, although TRAF6 is a downstream molecule for both RANK and IL-1R (IL-1 receptor), which suggests that RANK may also activate a TRAF6-independent signaling pathway to induce osteoclast differentiation.36
Recruitment of TRAF6 to RANK forms a signaling complex containing TGF-β-activated kinase (TAK) 1 and TAK-1-binding protein (TAB) 2 to activate all three mitogen-activated protein kinase (MAPK) pathways including ERK, JNK, and p38.37 The importance of TRAF6-dependent MAPK activation was confirmed by several studies. RANKL could not activate JNK and p38 in TRAF6-deficient spleen cells.33 A specific inhibitor of p38α and β (SB203580) suppressed RANKL-mediated osteoclast differentiation in RAW 264.7 cells, and osteoclast precursor cells derived from jnk1-lacking mice but not from jnk2-lacking mice exhibited reduced ability to differentiate to osteoclasts.3839 Based on a recent study, mice with genetic deletion of erk1 exhibited reduced osteoclast formation in vivo, suggesting that ERK1 plays an important role in osteoclast differentiation.40 RANKL activates ERK, JNK, and p38 through activation of MEK1/2, MKK7, and MKK6 to induce activation of their downstream targets such as c-Fos, AP-1 transcription factors, and MITF in osteoclast precursors, respectively.414243444546
RANKL also activates the PI3K/Akt pathway through TRAF6.8 In osteoclasts, activation of PI3K/Akt may be dependent on Src kinase activity, since a Src family kinase inhibitor or genetic deletion of c-Src inhibits RANKL-mediated Akt activation.8 Activated PI3K generates phosphatidylinositol-(3,4,5)-phosphate (PIP3) at the plasma membrane to recruit Akt.47 Two negative regulators of PIP3 production, phosphatase and tensin homolog (PTEN) and SH2-containing inositol phosphatase 1 (SHIP1), negatively regulate osteoclast differentiation. The PI3K inhibitor LY294002 also has an inhibitory effect on osteoclast formation.484950 Taken together, these studies show that the PI3K/Akt pathway is involved in osteoclast differentiation.
NF-κB is one of the important transcription factors for osteoclast differentiation that are activated by RANKL. NF-κB is activated through two pathways: classical and alternative. It has been shown that TRAF6 activates the classical NF-κB pathway, whereas TRAF2 and TRAF5 activate both classical and alternative NF-κB pathways.3751 Five protein groups—Rel, RelA, RelB, NF-κB1 (p50), and NF-κB2 (p52)—constitute NF-κB proteins, which are dimeric transcription factors that bind to κB sites. Among these protein groups, p50 and p52 lack a transcription activation domain and therefore require dimerization with other NF-κB proteins possessing a transcription activation domain, such as Rel, RelA, and RelB.5253 The importance of NF-κB proteins is recognized by p50/p52 double-knockout mice, in which osteoclastogenesis was prevented, although single knockout of p50 or p52 did not show a distinct phenotype.5455 In its inactive state, NF-κB exists as a complex with inhibitory κB protein (IκB) in the cytoplasm. Upon RANKL stimulation, IκB kinase (IKK) is activated; it phosphorylates IκB and mediates ubiquitin-dependent proteasomal degradation of IκB, and ultimately results in release of NF-κB from the NF-κB/IκB complex. NF-κB released from the NF-κB/IκB complex is now free to translocate from the cytoplasm to the nucleus and binds to its target genes.5253 IKK consists of a catalytic component (IKKα, IKKβ) and a regulatory component (IKKγ). Although both IKKα and IKKβ are involved in catalytic activity, IKKβ seems to function more critically as a catalytic component. Interruption of IKKα resulted in the disruption of osteoclast differentiation in vitro only, whereas interruption of IKKβ disrupted osteoclast differentiation both in vitro and in vivo, suggesting that IKKβ is critical for RANKL-mediated IκB degradation.56 An alternative pathway of NF-κB activation exists, in which RelB:p52 dimers are involved. In the alternative NF-κB activation pathway, NF-κB-inducing kinase (NIK) and IKKα are required for the formation of the RelB:p52 complex from p100.525356 Therefore, NIK plays an important role in the alternative pathway. However, osteopetrosis was not observed in NIK-deficient mice, suggesting that NIK-induced alternative NF-κB activation is not critical for NF-κB activation.57
RANKL signaling strongly induces NFATc1, which is a master transcription factor for the terminal differentiation of osteoclasts. RANKL activates NF-κB and c-Fos to stimulate induction of NFATc1 in the early phase of osteoclastogenesis.58 RANKL-mediated NFATc1 induction is impaired in both p50/p52-deficient and c-fos-deficient cells.5960 The activated c-Fos may cooperate with NFATc1 itself for vigorous induction of NFATc1 during terminal osteoclastogenesis.58 Thus, RANKL enhances NFATc1 transcription through the activation of two essential transcription factors for osteoclast differentiation, NF-κB and c-Fos, which in turn induce a self-sustaining positive autoregulatory system to maintain sufficient NFATc1 expression. NFATc1 regulates several osteoclast-specific genes including tartrate-resistant acid phosphatase (TRAP), osteoclast-associated receptor (OSCAR), and cathepsin K in cooperation with other transcription factors.3558616263 An essential role of NFATc1 in osteoclasts has been well established both in vitro and in vivo. In vitro, NFATc1-deficient embryonic stem cells failed to differentiate into osteoclasts, and ectopic expression of NFATc1 in osteoclast precursor cells induced osteoclast differentiation even in the absence of RANKL.35 The in vivo observation that deletion of NFATc1 in young mice results in osteopetrosis owing to impaired osteoclastogenesis also supported the important role of NFATc1 in osteoclasts.64
The activation of most NFAT transcription factor family members (NFATc1/c2/c3/c4) is originally regulated by calcium/ calmodulin signaling. In fact, since RANK does not seem to directly initiate calcium signaling and RANKL can only induce a partial activation of NFATc1 in osteoclast precursor cells, it has been suggested that costimulatory signaling for RANK may cooperate with RANKL to induce full activation of NFATc1 through calcium signaling pathways.35 It has been shown that tyrosine-based activation motif (ITAM)-bearing molecules such as DNAX-activating protein 12 (DAP12) and Fc receptor common γ chain (FcRγ) mediate calcium signaling and activate NFAT in immune cells.65 In osteoclasts, DAP12 and FcRγ also play an important role in the activation of NFATc1 through calcium signaling pathways. The severe osteopetrotic bone phenotype of mice doubly deficient in DAP12 and FcRγ suggests that immunoglobulin-like receptors associated with DAP12 and FcRγ are critical for osteoclast differentiation.910 DAP12 is associated with triggering receptor expressed in myeloid cells (TREM) 2 and signal-regulator protein β1 (SIRPβ1), whereas FcRγ interacts with OSCAR and paired immunoglobulin-like receptor (PIR-A) in osteoclasts.9 RANKL-mediated phosphorylation of ITAM through an unknown mechanism results in the activation of Syk and PLCγ. Activated PLCγ mobilizes intracellular calcium, which in turn activates the calmodulin-dependent phosphatase calcineurin. Calcineurin directly dephosphorylates serine residues in NFATc1, allowing for its rapid translocation into the nucleus and subsequent activation. Recently, specific tyrosine kinases that may provide a link between ITAM and RANK signaling were suggested. RANKL-mediated activation of Tec family tyrosine kinases such as BTK and Tec leads to phosphorylation of PLCγ to release calcium from the endoplasmic reticulum.66 Tec and BTK double-deficient mice develop an osteopetrotic phenotype, suggesting that these two kinases are associated with the regulation of osteoclastogenesis.66
Increased osteoclast formation is involved in bone diseases including osteoporosis and rheumatoid arthritis. Therefore, study of signaling pathways regulating osteoclast differentiation is crucial for a thorough understanding of the skeletal system in pathological conditions. Although significant signaling pathways for osteoclast differentiation have been elucidated, future studies of delicate regulatory networks involved in bone homeostasis are required for development of useful therapeutic strategies. Notably, future studies should focus on investigating the exact mechanisms underlying RANKL-mediated activation of costimulatory signals for RANK.
References
1. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7:292–304. PMID: 17380158.
2. Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006; 354:2250–2261. PMID: 16723616.
3. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–649. PMID: 12897775.
4. Sherr CJ. Colony-stimulating factor-1 receptor. Blood. 1990; 75:1–12. PMID: 2153029.
5. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999; 20:345–357. PMID: 10368775.
6. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176. PMID: 9568710.
7. Lum L, Wong BR, Josien R, Becherer JD, Erdjument-Bromage H, Schlöndorff J, et al. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem. 1999; 274:13613–13618. PMID: 10224132.
8. Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999; 4:1041–1049. PMID: 10635328.
9. Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004; 428:758–763. PMID: 15085135.
10. Mócsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004; 101:6158–6163. PMID: 15073337.
11. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990; 87:4828–4832. PMID: 2191302.
12. Marks SC Jr, Wojtowicz A, Szperl M, Urbanowska E, MacKay CA, Wiktor-Jedrzejczak W, et al. Administration of colony stimulating factor-1 corrects some macrophage, dental, and skeletal defects in an osteopetrotic mutation (toothless, tl) in the rat. Bone. 1992; 13:89–93. PMID: 1581113.
13. Xu F, Teitelbaum SL. Osteoclasts: New Insights. Bone Res. 2013; 1:11–26. PMID: 26273491.
14. Hamilton JA. CSF-I signal transduction: what is of functional significance? Immunol Today. 1997; 18:313–317. PMID: 9238832.
15. Feng X, Takeshita S, Namba N, Wei S, Teitelbaum SL, Ross FP. Tyrosines 559 and 807 in the cytoplasmic tail of the macrophage colony-stimulating factor receptor play distinct roles in osteoclast differentiation and function. Endocrinology. 2002; 143:4868–4874. PMID: 12446614.
16. Takeshita S, Faccio R, Chappel J, Zheng L, Feng X, Weber JD, et al. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J Biol Chem. 2007; 282:18980–18990. PMID: 17420255.
17. Yu W, Chen J, Xiong Y, Pixley FJ, Dai XM, Yeung YG, et al. CSF-1 receptor structure/function in MacCsf1r-/- macrophages: regulation of proliferation, differentiation, and morphology. J Leukoc Biol. 2008; 84:852–863. PMID: 18519746.
18. Yu W, Chen J, Xiong Y, Pixley FJ, Yeung YG, Stanley ER. Macrophage proliferation is regulated through CSF-1 receptor tyrosines 544, 559, and 807. J Biol Chem. 2012; 287:13694–13704. PMID: 22375015.
19. Alonso G, Koegl M, Mazurenko N, Courtneidge SA. Sequence requirements for binding of Src family tyrosine kinases to activated growth factor receptors. J Biol Chem. 1995; 270:9840–9848. PMID: 7730365.
20. Lee AW, States DJ. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol Cell Biol. 2000; 20:6779–6798. PMID: 10958675.
21. Xiong Y, Song D, Cai Y, Yu W, Yeung YG, Stanley ER. A CSF-1 receptor phosphotyrosine 559 signaling pathway regulates receptor ubiquitination and tyrosine phosphorylation. J Biol Chem. 2011; 286:952–960. PMID: 21041311.
22. Bourette RP, Myles GM, Choi JL, Rohrschneider LR. Sequential activation of phoshatidylinositol 3-kinase and phospholipase C-gamma2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J. 1997; 16:5880–5893. PMID: 9312046.
23. Mancini A, Niedenthal R, Joos H, Koch A, Trouliaris S, Niemann H, et al. Identification of a second Grb2 binding site in the v-Fms tyrosine kinase. Oncogene. 1997; 15:1565–1572. PMID: 9380408.
24. Bourette RP, Rohrschneider LR. Early events in M-CSF receptor signaling. Growth Factors. 2000; 17:155–166. PMID: 10705574.
25. Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002; 99:111–120. PMID: 11756160.
26. Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997; 390:175–179. PMID: 9367155.
27. Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999; 13:2412–2424. PMID: 10500098.
28. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999; 397:315–323. PMID: 9950424.
29. Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 2003; 14:251–263. PMID: 12787563.
30. Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Characterization of the intracellular domain of receptor activator of NFkappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem. 1998; 273:20551–20555. PMID: 9685412.
31. Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999; 13:1015–1024. PMID: 10215628.
32. Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999; 4:353–362. PMID: 10421844.
33. Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, et al. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001; 20:1271–1280. PMID: 11250893.
34. Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem. 1998; 273:28355–28359. PMID: 9774460.
35. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901. PMID: 12479813.
36. Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000; 97:1566–1571. PMID: 10677500.
37. Mizukami J, Takaesu G, Akatsuka H, Sakurai H, Ninomiya-Tsuji J, Matsumoto K, et al. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol. 2002; 22:992–1000. PMID: 11809792.
38. Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, et al. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology. 2002; 143:3105–3113. PMID: 12130576.
39. David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci. 2002; 115:4317–4325. PMID: 12376563.
40. He Y, Staser K, Rhodes SD, Liu Y, Wu X, Park SJ, et al. Erk1 positively regulates osteoclast differentiation and bone resorptive activity. PLoS One. 2011; 6:e24780. PMID: 21961044.
41. Yamamoto A, Miyazaki T, Kadono Y, Takayanagi H, Miura T, Nishina H, et al. Possible involvement of IkappaB kinase 2 and MKK7 in osteoclastogenesis induced by receptor activator of nuclear factor kappaB ligand. J Bone Miner Res. 2002; 17:612–621. PMID: 11918218.
42. Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J Biol Chem. 2000; 275:31155–31161. PMID: 10859303.
43. Kashiwada M, Shirakata Y, Inoue JI, Nakano H, Okazaki K, Okumura K, et al. Tumor necrosis factor receptor-associated factor 6 (TRAF6) stimulates extracellular signal-regulated kinase (ERK) activity in CD40 signaling along a ras-independent pathway. J Exp Med. 1998; 187:237–244. PMID: 9432981.
44. Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995; 20:117–122. PMID: 7709430.
45. Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994; 266:443–448. PMID: 7939685.
46. Mansky KC, Sankar U, Han J, Ostrowski MC. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-kappa B ligand signaling. J Biol Chem. 2002; 277:11077–11083. PMID: 11792706.
47. Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000; 346 Pt 3:561–576. PMID: 10698680.
48. Sugatani T, Alvarez U, Hruska KA. PTEN regulates RANKL- and osteopontin-stimulated signal transduction during osteoclast differentiation and cell motility. J Biol Chem. 2003; 278:5001–5008. PMID: 12460992.
49. Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ, et al. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med. 2002; 8:943–949. PMID: 12161749.
50. Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee SY, et al. Akt induces osteoclast differentiation through regulating the GSK3β/ NFATc1 signaling cascade. J Immunol. 2012; 188:163–169. PMID: 22131333.
51. Hauer J, Püschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, et al. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A. 2005; 102:2874–2879. PMID: 15708970.
52. Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002; 109:S81–S96. PMID: 11983155.
53. Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004; 18:2195–2224. PMID: 15371334.
54. Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997; 11:3482–3496. PMID: 9407039.
55. Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997; 3:1285–1289. PMID: 9359707.
56. Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu LC, Cao Y, et al. I{kappa}B kinase (IKK){beta}, but not IKK{alpha}, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005; 201:1677–1687. PMID: 15897281.
57. Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003; 198:771–781. PMID: 12939342.
58. Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005; 202:1261–1269. PMID: 16275763.
59. Li F, Matsuo K, Xing L, Boyce BF. Over-expression of activated NFATc1 plus RANKL rescues the osteoclastogenesis defect of NF-κB p50/p52 double knockout splenocytes. J Bone Miner Res. 2004; 19:S2. PMID: 15505937.
60. Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004; 279:26475–26480. PMID: 15073183.
61. Kim K, Kim JH, Lee J, Jin HM, Lee SH, Fisher DE, et al. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem. 2005; 280:35209–35216. PMID: 16109714.
62. Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005; 280:32905–32913. PMID: 16046394.
63. Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004; 279:45969–45979. PMID: 15304486.
64. Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008; 118:3775–3789. PMID: 18846253.
65. Pitcher LA, van Oers NS. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003; 24:554–560. PMID: 14552840.
66. Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008; 132:794–806. PMID: 18329366.
FIG. 1
Osteoclast differentiation is stimulated by M-CSF and RANKL. M-CSF induces the proliferation and survival of osteoclast precursor cells through activation of ERK and Akt. RANKL recruits TRAF6 to activate MAPKs, Akt, and NFATc1 to promote differentiation of osteoclast precursors to osteoclasts. In addition to RANKL signaling, costimulatory signaling provides robust NFATc1 induction through activation of calcium signaling.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download