Abstract
Gestational diabetes mellitus (GDM) is an important disease which complicates pregnant woman and fetus. Large for gestational age (LGA) is one of the primary complications and is closely associated with the hyperglycemia of pregnant woman. Although strict control of blood glucose can decrease the occurrence of LGA, the rate of LGA in GDM pregnancy is higher than that of normal pregnancy. Understanding of the difference of fetal growth between LGA and adequate for gestational age in GDM pregnancy and consideration about the time and marker for prediction and prevention of LGA in GDM pregnancy are helpful for prenatal care of GDM pregnancy. In this article, the prediction and prevention of LGA in GDM pregnancy will be discussed.
REFERENCES
1). Lee HN., Lee GSR., Kim SJ., Rho SH., Baik EJ., Kang BC, et al. Clinical study of maternal and perinatal complication in pregnancy with diabetes mellitus. Korean J Obstet Gynecol. 1999. 42:2712–9.
2). Yang SC., Kim HS., Yang JI., Lee HJ., Ahn ST., Seo SS, et al. Study of the diagnostic criteria for gestational diabetes mellitus. Korean J Obstet Gynecol. 2002. 45:1932–9.
3). Metzger BE., Lowe LP., Dyer AR., Trimble ER., Chaovarindr U., Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. HAPO Study Cooperative Research Group. N Engl J Med. 2008. 358:1991–2002.
4). Crowther CA., Hiller JE., Moss JR., McPhee AJ., Jeffries WS., Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005. 352:2477–86.
5). Landon MB., Spong CY., Thom E., Carpenter MW., Ramin SM., Casey B, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009. 361:1339–48.
6). Langer O., Rodriguez DA., Xenakis EM., McFarland MB., Berkus MD. ArrendondoF. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1995. 172:1642–3.
7). Langer O., Levy J., Brustman L., Anyaegbunam A., Merdatz R., Divon M. Glycemic control in gestational diabetes mellitus: how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol. 1989. 161:646–53.
8). Sermer M., Naylor CD., Gare DJ., Kenshole AB., Ritchie JW., Farine D, et al. Impact of increasing carbohydrate intolerance on maternal fetal outcomes in 3,637 women without gestational diabetes. Am J Obstet Gynecol. 1995. 173:146–56.
9). Brans YW., Shannon DL., Hunter MA. Maternal diabetes and neonatal macrosomia. II. Neonatal anthropometric measurements. Early Hum Dev. 1983. 8:297–305.

10). Ogata ES., Sabbagha R., Metzger BE., Phelps RL., Depp R., Freinkel N. Serial ultrasonography to assess evolving fetal macrosomia. Studies in 23 pregnant diabetic women. JAMA. 1980. 243:2405–8.

11). Landon MB., Mintz MC., Gabbe SG. Sonographic evaluation of fetal abdominal growth: predictor of the large-for-gestational-age infant in pregnancies complicated by diabetes mellitus. Am J Obstet Gynecol. 1989. 160:115–21.

12). Weiss PAM. Gestational diabetes: a survey and the Graz approach to diagnosis and therapy. Weiss P, Coustan D, editors. editors.Gestational Diabetes. Vienna: Springer-Verlag;1988. p. 1–55.

13). Burkhart W., Holzgreve W., Cane WR., Schneider HPG. Antenatal assessment of fetal outcome in pregnant diabetes. J Perinat Med. 1986. 14:293–7.
14). Carpenter M., Canick JA., Hogan JW., Shellum C., Somers M., Star JA. Amniotic fluid insulin at 14-20 weeks' gestation: association with later maternal glucose intolerance and birth macrosomia. Diabetes Care. 2001. 24:1259–63.
15). Catalano PM., Tyzbir ED., Allen SR., McBean JH., McAuliffe TL. Evaluation of fetal growth by estimation of neonatal body composition. Obstet Gynecol. 1997. 176:28–32.
16). Bernstein IM., Goran MI., Amini SB., Catalano PM. Differential growth of fetal tissues during the second half of pregnancy. Am J Obstet Gynecol. 1997. 176:28–32.

17). Winn HN., Holcomb WL. Fetal non-muscular soft tissue: a prenatal assessment. J Ultrasound Med. 1993. 4:197–99.

18). Gardeil F., Greene R., Stuart B., Turner MJ. Subcutaneous fat in the fetal abdomen as a predictor of growth restriction. Obstet Gynecol. 1999. 94:209–12.

19). Larciprete G., Valensise H., Vasapollo B., Novelli GP., Parretti E., Altomare F, et al. Fetal subcutaneous tissue thickness (SCTT) in healthy and gestational diabetic pregnancies. Ultrasound Obstet Gynecol. 2003. 22:591–7.

20). Jang HC., Park JE., Yin CH., Chung HY., Han KO., Yoon HK, et al. Fetal hyperinsulinemia and ultrasonographic measurement of fetal growth in pregnancy complicated by gestational diabetes mellitus. J Korean Diabetes Assoc. 1999. 23:506–17.
21). Rosati P., Exacoustos C., Caruso A., Mancuso S. Ultrasound diagnosis of fetal macrosomia. Ultrasound Obstet Gynecol. 1991. 2:23–90.

22). Skovron ML., Berkowits GS., Lapinski RJ., Kim JM., Chitkara U. Evlauation of early third-trimester ultrasound screening for intrauterine growth retardation. J Ultrasound Med. 1991. 10:153–9.
23). O'Reilly-Green C., Divon M. Sonographic and clinical method in the diagnosis of macromia. Clin Obstet Gynecol. 2000. 43:309–20.
24). Schaefer-Graf U., Buhling K., Kjos SL., Dudenhausen JW., Vetter K. Amniotic fluid insulin levels and fetal abdominal circumference at time of amniocentesis in pregnancies with diabetes. Diabet Med. 2003. 20:349–54.

25). Gojnic M., Stefanovic T., Perovic M., Arsic B., Garalejic E., Micic J, et al. Prediction of fetal macrosomia with ultrasound parameters and maternal glycemic controls in gestational diabetes mellitus. Clin Exp Obstet Gynecol. 2012. 39:512–5.
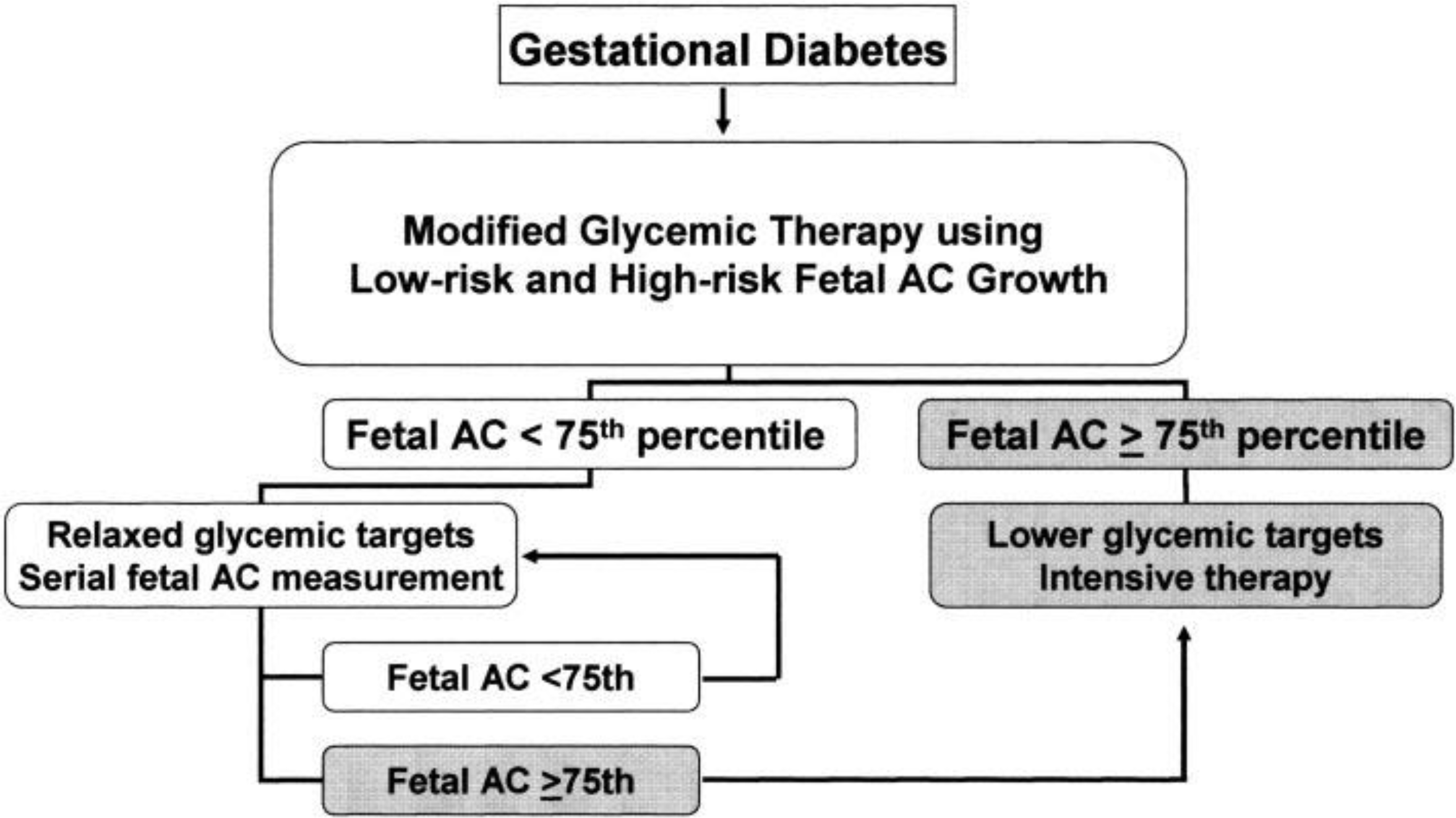
26). Kjos RL., Schaefer-Graf UM. Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic targets. Diabetes Care. 2007. 30(Suppl 2):S200–5.

27). Buchanan TA., Kjos SL., Montoro M., Wu PY., Madrilejo NG., Gonzalez M, et al. Use of fetal ultrasound to select metabolic therapy for pregnancies complicated by mild gestational diabetes. Diabetes Care. 1994. 17:275–83.

28). Kjos SL., Schaefer-Graf U., Sardesi S., Peters RK., Buley A., Xiang AH, et al. A randomized controlled trial utilizing glycemic puls fetal ultrasound parameters vs. glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care. 2001. 24:1904–10.
29). Schaefer-Graf UM., Kjos SL., Fauz R., Kuehling KJ., Sierbert G., Buhrer C, et al. A randomized trial investigating the utility of a predominantly fetal-growth based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care. 2004. 27:297–302.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download