Abstract
Purpose:
Perinatal hypoxic-ischemic (HI) brain injury remains a common cause of chronic handicapping conditions of cerebral palsy, mental retardation, learning disability, and epilepsy. HI brain injury induces cell death via either necrosis or apoptosis. Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family. It plays key roles in survival, differentiation, and maintenance of neurons. This study was to investigate the neuroprotective effects of BDNF via the mechanisms of anti-apoptosis in HI brain injury by using cortical astrocyte and neuronal cell culture.
Methods:
Cortical astrocytes culture of 1-day-old Sprague-Dawley (SD) rat pups and embryonic cortical neuronal cell culture of SD rats at 14-day gestation were done. The Normoxia group was prepared in 5% CO2 incubators and the Hypoxia group and Hypoxia+BDNF group (after treatment with BDNF for 24 hours) were placed in 1% O2 incubators (94% N2, 5% CO2) for 6 or 18 hours. The expression of Bcl-2 and Bax were assessed by real-time PCR and western blot. The caspase-3 activation was evaluated by caspase activity assay kit.
Results:
In astrocyte and neuronal cell, the expressions of Bcl-2 in the hypoxia groups were reduced compared to the normoxia groups, whereas, those in the Hypoxia+BDNF groups were increased compared to the hypoxia groups. However, the expressions of Bax and caspase-3 and the ratio of Bax/Bcl-2 were revealed reversely. In astrocyte, Hypoxia group for 6 hours was not significantly altered in Bcl-2, Bax expressions.
REFERENCES
1). Kaltschmidt C., Kaltschmidt B., Neumann H., Wekerle H., Baeuerle PA. Constitutive NF-kappa B activity in neurons. Mol Cell Biol. 1994. 14:3981–92.

2). Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001. 7:56–64.

4). Lamigeon C., Bellier JP., Sacchettoni S., Rujano M., Jacque-mont B. Enhanced neuronal protection from oxidative stress by coculture with glutamic acid decarboxylase-expressing astrocytes. J Neurochem. 2001. 77:598–606.

5). Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991. 14:453–501.

6). Lalier L., Cartron PF., Juin P., Nedelkina S., Mano S., Bechinger B, et al. Bax activation and mitochondrial insertion during apoptosis. Apoptosis. 2007. 12:887–96.

7). Oltvai Z. N, Milliman CL, Korsmeyer SJ. Bcl-2 hetero-dimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993. 74:609–19.
8). Takada N., Yamaguchi H., Shida K., Terajima D., Satou Y., Kasuya A, et al. The cell death machinery controlled by Bax and Bcl-XL is evolutionarily conserved in Ciona intestinalis. Apoptosis. 2005. 10:1211–20.

9). Han BH., D'Costa A., Back SA., Parsadanian M., Patel S., Shah AR, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000. 7:38–53.
10). Adamec E., Yang F., Cole GM., Nixon RA. Multiple-label immunocytochemistry for the evaluation of nature of cell death in experimental models of neurodegeneration. Brain Res Brain Res Protoc. 2001. 7:193–202.

11). Cheng Y., Deshmukh M., D'Costa A., Demaro JA., Gidday J., Shah A, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxi-cischemic brain injury. J Clin Invest. 1998. 101:1992–9.

12). Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006. 361:1545–64.

13). Tapia-Arancibia L., Rage F., Givalois L., Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004. 25:77–107.
15). Schäbitz WR., Sommer C., Zoder W., Kiessling M., Schwaninger M., Schwab S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000. 31:2212–7.

16). Cheng Y., Gidday JM., Yan Q., Shah AR., Holtzman DM. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997. 41:521–9.

17). Liu YJ., Zhuang J., Zhu HY., Shen YX., Tan ZL., Zhou JN. Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J Pineal Res. 2007. 43:232–8.

18). Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci. 1997. 71:143–55.

19). Callahan DJ., Engle MJ., Volpe JJ. Hypoxic injury to developing glial cells: protective effect of high glucose. Pediatr Res. 1990. 27:186–90.

20). Hong SS., Gibney GT., Esquilin M., Yu J., Xia Y. Effect of protein kinases on lactate dehydrogenase activity in cortical neurons during hypoxia. Brain Res. 2004. 1009:195–202.

21). Arancibia S., Silhol M., Moulière F., Meffre J., Höllinger I., Maurice T, et al. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008. 31:316–26.

22). Simonian NA., Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996. 36:83–106.

23). Dirnagl U., Iadecola C., Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999. 22:391–7.

24). Sauvageot CM., Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002. 12:244–9.

25). Garcia CM., Darland DC., Massingham LJ., D'Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res. 2004. 152:25–38.
27). Nedergaard M., Ransom B., Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003. 26:523–30.

29). Olney JW., Tenkova T., Dikranian K., Muglia LJ., Jermako-wicz WJ., D'Sa C, et al. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002. 9:205–19.

30). Merry DE., Veis DJ., Hickey WF., Korsmeyer SJ. bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development. 1994. 120:301–11.

31). Vekrellis K., McCarthy MJ., Watson A., Whitfield J., Rubin LL., Ham J. Bax promotes neuronal cell death and is down-regulated during the development of the nervous system. Development. 1997. 124:1239–49.

32). Parikh N., Koshy C., Dhayabaran V., Perumalsamy LR., Sowdhamini R., Sarin A. The N-terminus and alpha-5, alpha-6 helices of the pro-apoptotic protein Bax, modulate functional interactions with the anti-apoptotic protein Bcl-xL. BMC Cell Biol. 2007. 8:16.

33). Chen J., Graham SH., Nakayama M., Zhu RL., Jin K., Stetler RA, et al. Apoptosis repressor genes Bcl-2 and Bcl-x-long are expressed in the rat brain following global ischemia. J Cereb Blood Flow Metab. 1997. 17:2–10.

34). Hara A., Iwai T., Niwa M., Uematsu T., Yoshimi N., Tanaka T, et al. Immunohistochemical detection of Bax and Bcl-2 proteins in gerbil hippocampus following transient forebrain ischemia. Brain Res. 1996. 711:249–53.

35). Almli CR., Levy TJ., Han BH., Shah AR., Gidday JM., Holtzman DM. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000. 166:99–114.

36). Koh JY., Gwag BJ., Lobner D., Choi DW. Potentiated necrosis of cultured cortical neurons by neurotrophins. Science. 1995. 268:573–5.

37). Miyata K., Omori N., Uchino H., Yzmafuchi T., Isshiki A., Shibasaki F. Involvement of the brain-derived neurotrophic factor/TrkB pathway in neuroprotective effect of cyclosporine A in forebrain ischemia. Neuroscience. 2001. 105:571–8.
38). Barnabé-Heider F., Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003. 23:5149–60.

Fig. 1
Fluorescence images (x 200) of rat cortical astrocyte and neuronal cell cultured for 10 days and stained for the appropriate phenotypic markers. Nuclei were stained with DAPI (blue). Approximately 95% of the cells stain positive for astrocyte marker GFAP (green) (A), more than 95% of the cells are positive for the neuronal cell marker MAP2 (green) (B).
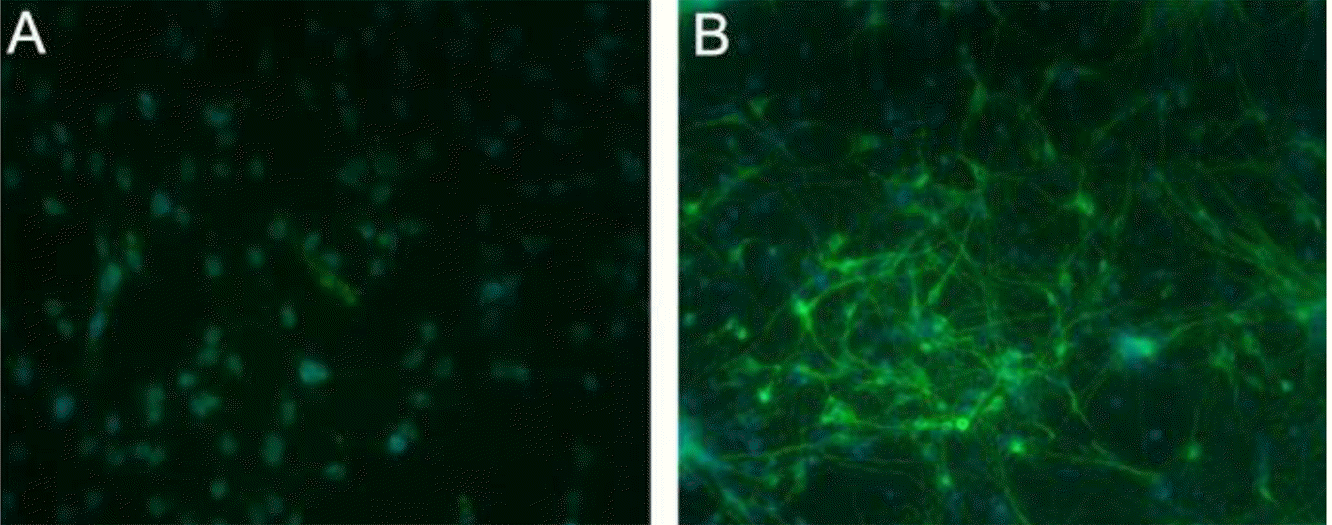
Fig. 2
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl-tetrazolium bromide (MTT) assay. Cultured dispersed astrocytes or neuronal cells were prepared with different concentrations of brain-derived neurotrophic factor (BDNF) for 24 hours before a hypoxic insult for 6 or 18 hours. The concentration of drug was 5, 100, and 200 ng/mL. The damaged cells were restored following administration of BDNF. The effective doses were 100 ng/mL in the HB groups both astrocyte and neuronal cell. N, normoxia; 6H, hypoxia for 6 hours; 6HB; hypoxia for 6 hours after treatment with BDNF; 18H, hypoxia for 18 hours; 18HB; hypoxia for 18 hours after treatment with BDNF. ∗P<0.05, statistically significant vs. N.
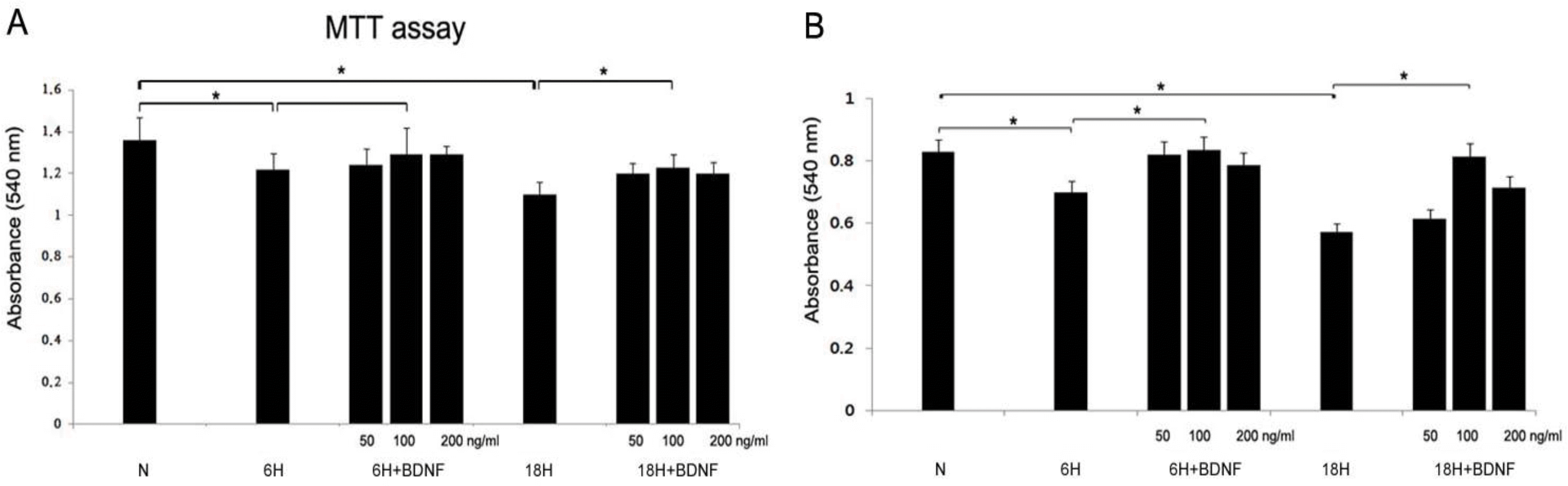
Fig. 3
Real-time PCRs of Bcl-2 (A; N, 100±5.0; 6H, 107.2±5.3; 6HB, 112.9±5.6; 18H, 52.3±2.6; 18HB, 73.2±3.7) and Bax (B; N, 100±2.0; 6H, 102.1±2.9; 6HB, 103.8±2.1; 18H, 115.6±1.7; 18HB, 113.7±3.0) mRNAs and the ratio of Bax/ Bcl-2 were revealed in the cortical astrocyte culture. BDNF was administered at 100 ng/mL. N, normoxia; 6H, hypoxia for 6 hours; 6HB; hypoxia for 6 hours after treatment with BDNF; 18H, hypoxia for 18 hours; 18HB; hypoxia for 18 hours after treatment with BDNF. ∗P<0.05, statistically significant vs. H.
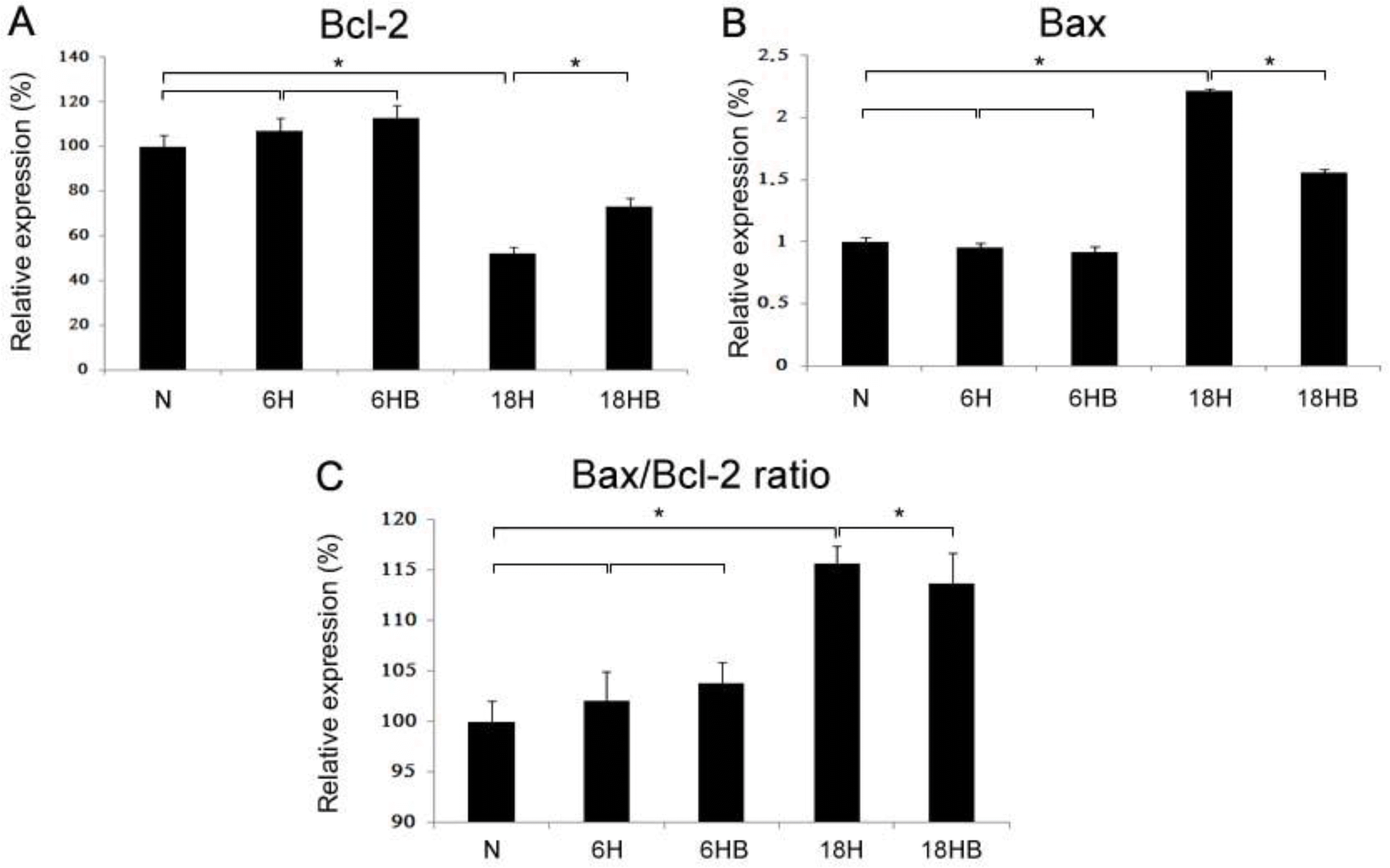
Fig. 4
Real-time PCRs of Bcl-2 (A; N, 100±4.5; 6H, 42.3±6.7; 6HB, 76.3±3.9; 18H, 30.8±4.0; 18HB, 62.9±5.5), Bax (B; N, 100±3.7; 6H, 121.0±5.2; 6HB, 95.9±7.1; 18H, 173.5±2.9; 18HB, 126.1±6.4) mRNAs and the ratio of Bax/Bcl-2 were revealed in the embryonic cortical neuronal cell culture. BDNF was administered at 100 ng/mL. N, normoxia H, hypoxia for 6 hours; 6HB; hypoxia for 6 hours after treatment with BDNF; 18H, hypoxia for 18 hours; 18HB; hypoxia for 18 hours after treatment with BDNF. ∗P<0.05, statistically significant vs. H.
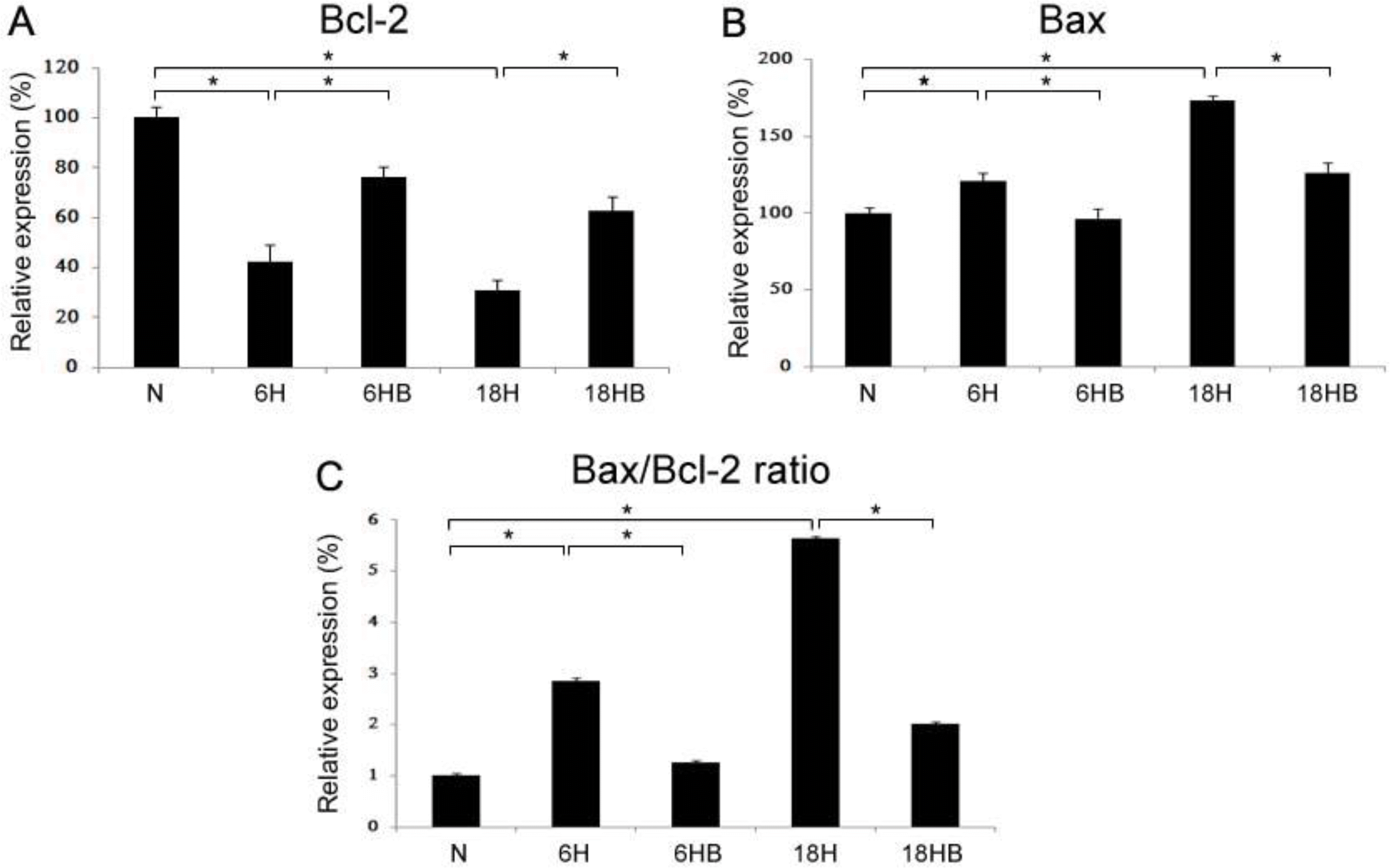
Fig. 5
Western blots (A) of Bcl-2 (B; N, 100±2.0; 6H, 94.9±1.8; 6HB, 98.0±0.6; 18H, 75.4±0.73; 18HB, 89.8±2.1) and Bax (C; N, 100±5.5; 6H, 105.8±4.8; 6HB, 102.5±4.2; 18H, 151.5±6.1; 18HB, 111.9±5.6) and the ratio of Bax/Bcl-2 were revealed in the cortical astrocyte culture (n=4). BDNF was administered at 100 ng/mL. N, normoxia; 6H, hypoxia for 6 hours; 6HB; hypoxia for 6 hours after treatment with BDNF; 18H, hypoxia for 18 hours; 18HB; hypoxia for 18 hours after treatment with BDNF. ∗P<0.05, statistically significant vs. H.
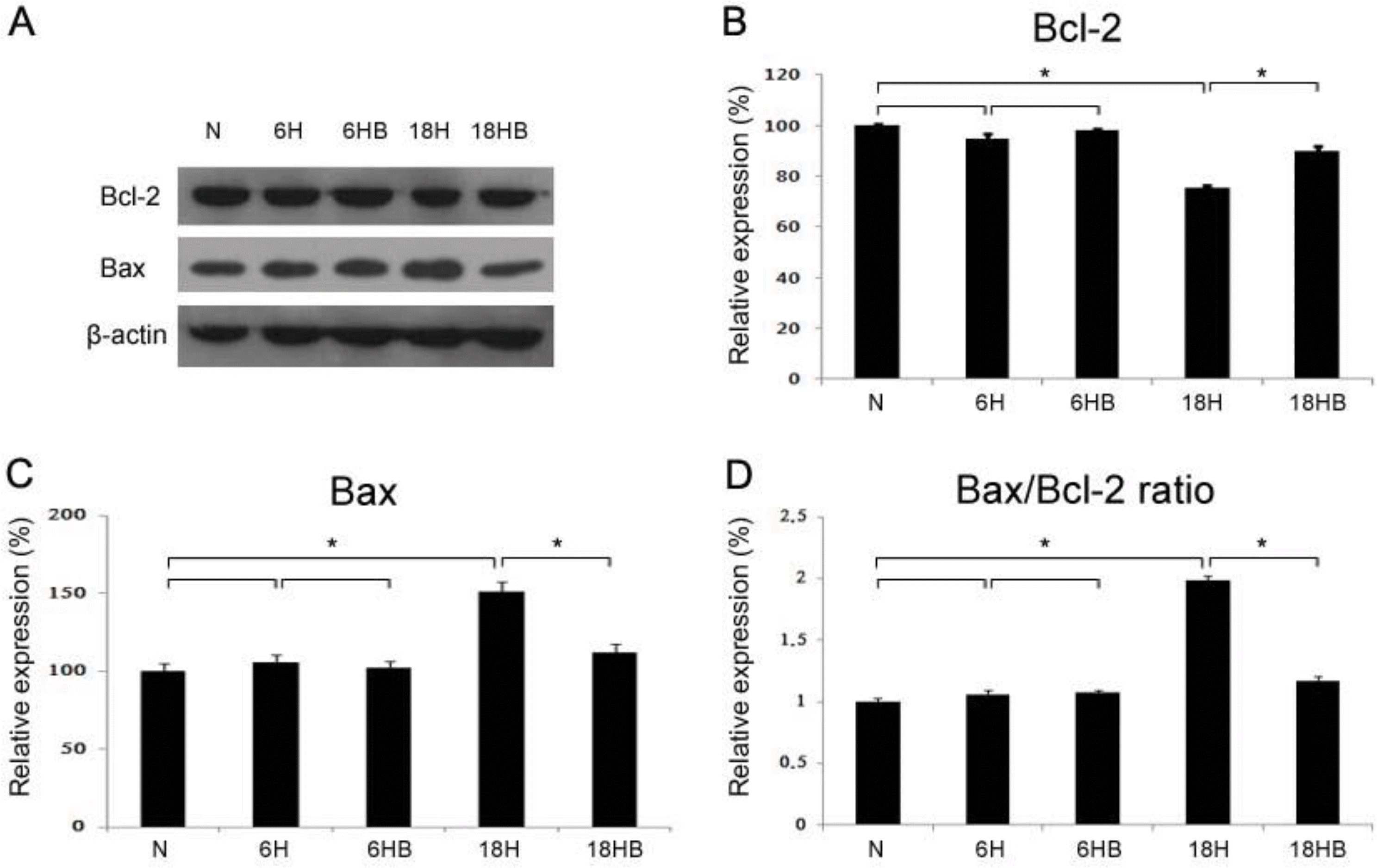
Fig. 6
Western blots (A) of Bcl-2 (B; N, 100±2.0; 6H, 42.1±3.2; 6HB, 82.3±1.9; 18H, 30.9±4.9; 18HB, 70.4±5.2), Bax (C; N, 100±5.7; 6H, 145.6±6.9; 6HB, 105.5±7.2; 18H, 164.3±8.1; 18HB, 121.9±3.7) and the ratio of Bax/Bcl-2 were revealed in the embryonic cortical neuronal cell culture (n=4). BDNF was administered at 100 ng/mL. N, normoxia; 6H, hypoxia for 6 hours; 6HB; hypoxia for 6 hours after treatment with BDNF; 18H, hypoxia for 18 hours; 18HB; hypoxia for 18 hours after treatment with BDNF. ∗P<0.05, statistically significant vs. H.
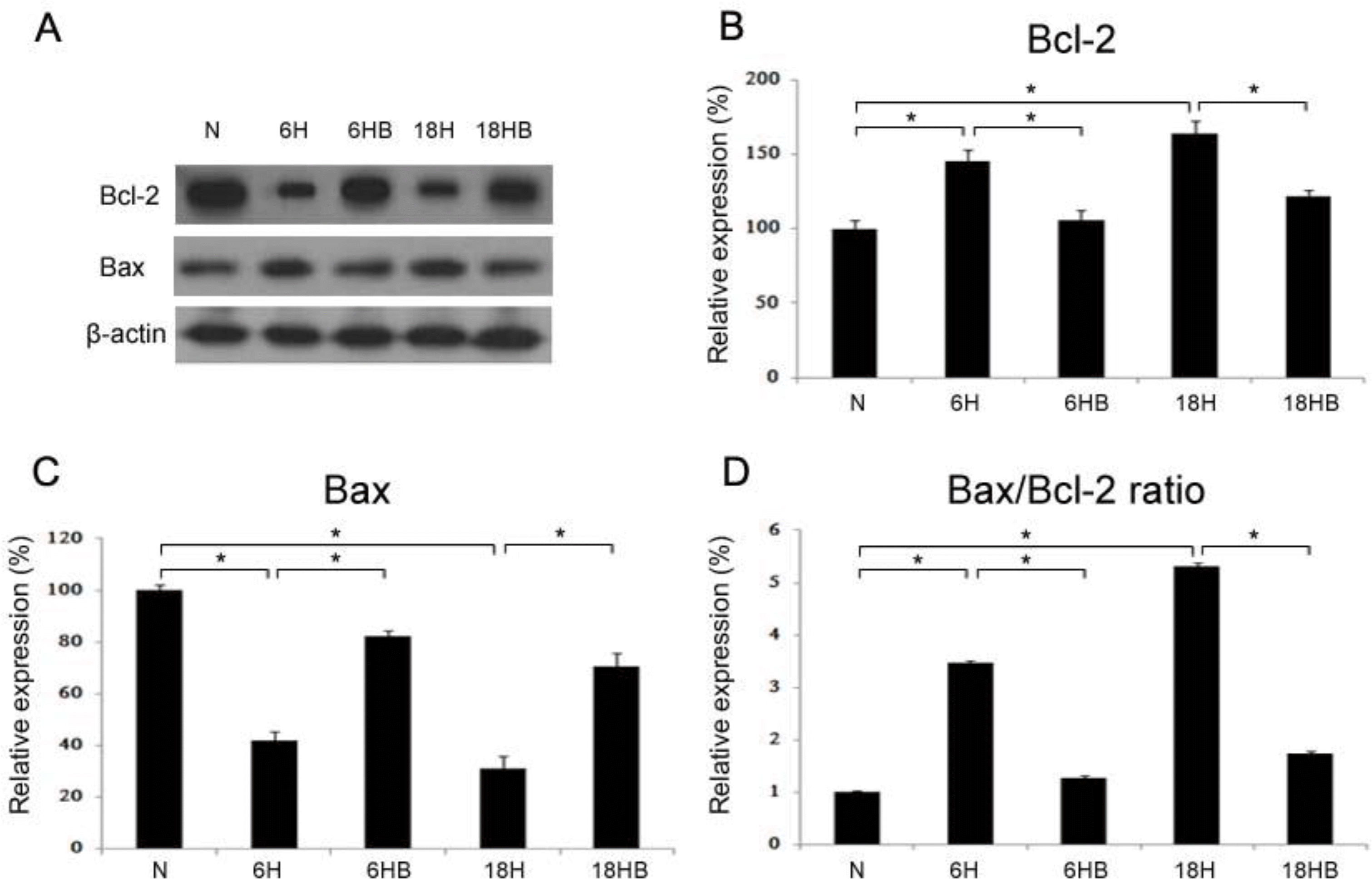
Fig. 7
Astrocyte (A) and neuronal cell (B) were plated dish 24 hours before the induction of apoptosis. After treatment with 100 ng/mL BDNF for 24 hours before a hypoxic insult, the activity of caspase-3 was assayed using a caspase-3/CPP32 colorimetric assay kit. ∗P<0.05, statistically significant vs. N.
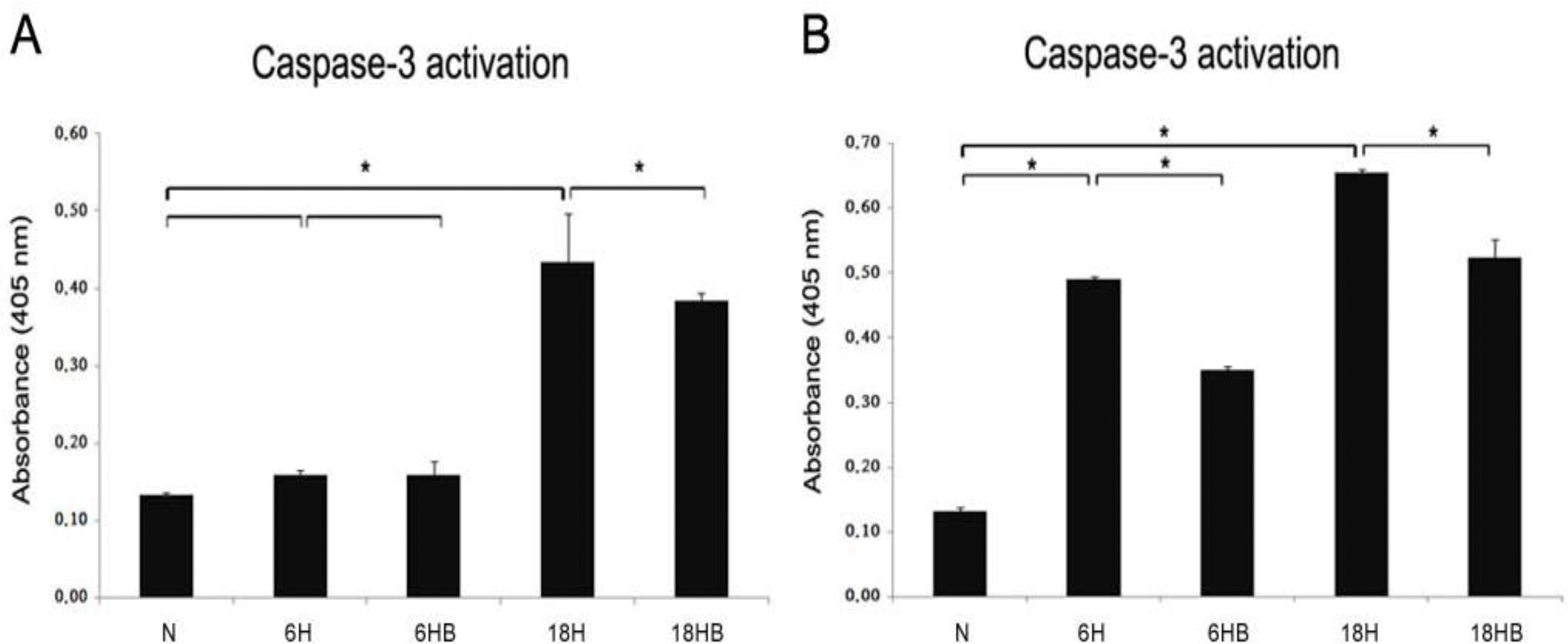
Table 1.
Primer Pairs and Annealing Temperatures for Real-Time PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download