Abstract
Purpose
Hypertensive disorders of pregnancy increase morbidity and mortality of fetus and neonates. Recently some studies revealed that antihypertensive agents affected the neonatal outcomes. The aim of this study was to investigate the prognosis of preterm infants delivered from the mothers with hypertensive disorders who were treated with antihypertensive agents and magnesium sulfate.
Methods
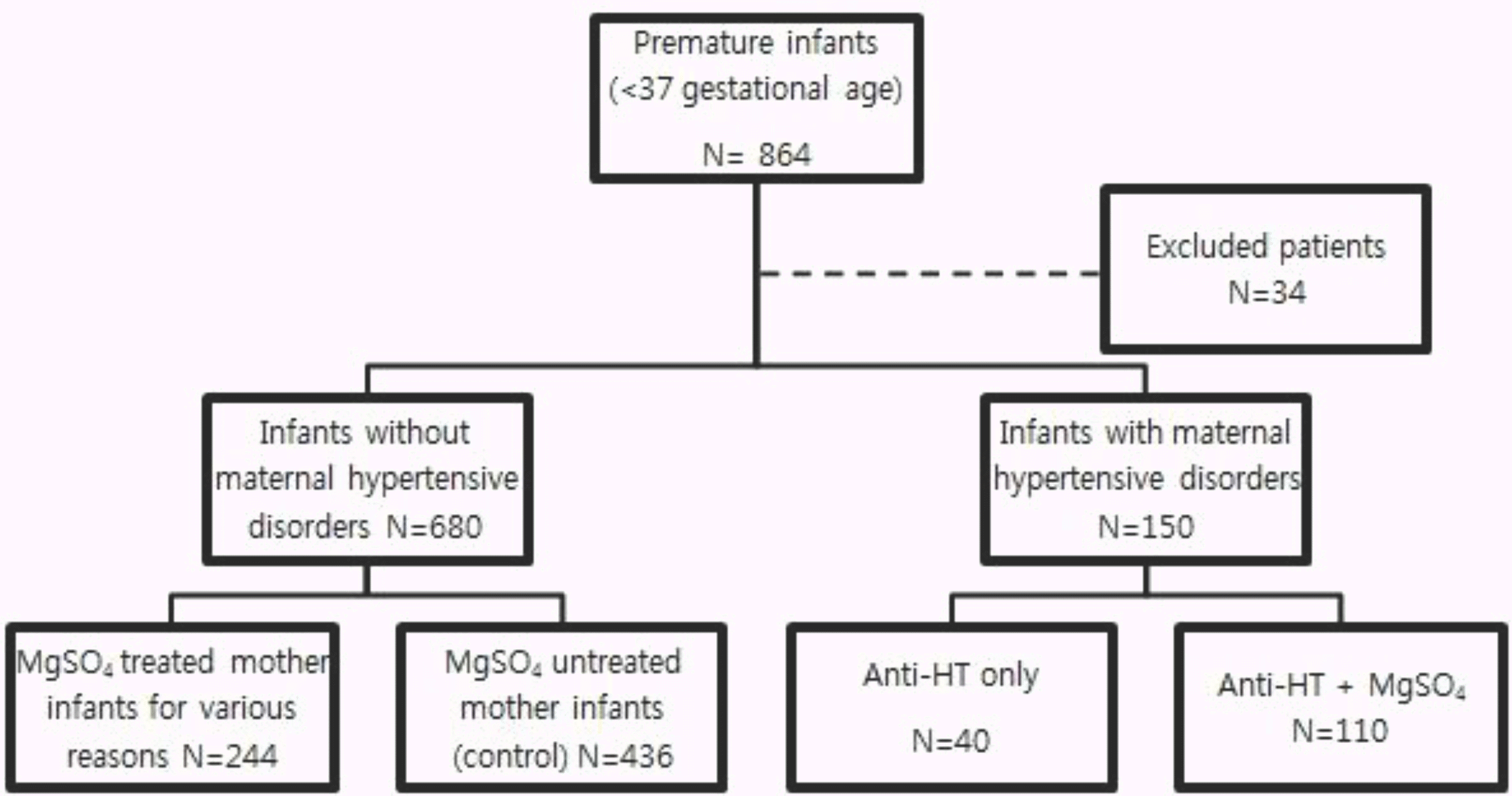
This retrospective study was conducted on preterm infants who were delivered from normotensive mother (control, n=436) and antihypertensive drugs +/– magnesium sulfate treated mother (study, n=150) between January 2009 and December 2013. Study group were divided into two groups based on whether they received antihypertensive drugs only (n=110) and additional magnesium sulfate (n=40). We compared the characteristics of mothers and neonatal outcomes.
Results
Study group had shorter gestational age (32.2±3.5 weeks vs. 33.7±3.0 weeks, P=0.000) and lower birth weight (1,810.5 ± 689.2 g, 2,212.1 ± 604.9 g, P=0.000), and higher rate of small for gestational age infants (22% vs 11%, P=0.000). One minute and 5 minutes Apgar score were lower, but duration of hospital days, oxygen supplement and mechanical ventilation were longer in study group. Respiratory distress syndrome, bronchopulmonary dysplasia, patent ductus arteriosus, retinopathy of prematurity, intraventricular hemorrhage occurred more in study group than control. The group treated with magnesium sulfate together with antihypertensive agent had lower 1 minute and 5 minutes Apgar score than the group taken antihypertensive agent only.
Conclusions
Mothers with hypertensive disorders have increased the risk of preterm delivery, low birth weight, and high neonatal morbidity rate. Therefore it is important to predict and manage possible complication. Moreover, if magnesium sulfate is taken, careful neonatal monitoring is needed because of possible low Apgar score.
Go to : 
References
1. Gofton EN, Capewell V, Natale R, Gratton RJ. Obstetrical intervention rates and maternal and neonatal outcomes of women with gestational hypertension. Am J Obstet Gynecol. 2001; 185:798–803.

2. Rugolo LMSS, Bentlin MR, Trindade CEP. Preeclampsia: effect on the fetus and newborn. NeoReviews. 2011; 12:198206.
3. Maloney KF, Heller D, Baergen RN. Types of maternal hypertensive disease and their association with pathologic lesions and clinical factors. Fetal Pediatr Pathol. 2012; 31:31923.

4. Report of the national high blood pressure education program working group on high blood pressure in pregnancy [editorial]. Am J Obstet Gynecol. 2000; 183:S1–22.
5. CindrovaDavies T. Gabor Than Award Lecture 2008: preeclampsia – from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009; 30(Suppl A):S55–65.

6. Al Khaja KA, Sequeira RP, Alkhaja AK, Damanhori AH. Drug treatment of hypertension in pregnancy: a critical review of adult guideline recommendations. J Hypertens. 2014; 32:454–63.
7. Easterling TR. Pharmacological management of hypertension in pregnancy. Semin Perinatol. 2014; 38:487–95.

8. Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004; 190:1520–6.

9. Klauser CK, Briery CM, Keiser SD, Martin RW, Kosek MA, Morrison JC. Effect of antenatal tocolysis on neonatal outcomes. J Matern Fetal Neonatal Med. 2012; 25:2778–81.

10. Abbassi-Ghanavati M, Alexander JM, McIntire DD, Savani RC, Leveno KJ. Neonatal effects of magnesium sulfate given to the mother. Am J Perinatol. 2012; 29:795–9.

11. Riaz M, Porat R, Brodsky NL, Hurt H. The effects of maternal magnesium sulfate treatment on newborns: a prospective controlled study. J Perinatol. 1998; 18:449–54.
12. Heida KY, Zeeman GG, Van Veen TR, Hulzebos CV. Neonatal side effects of maternal labetalol treatment in severe preeclampsia. Early Hum Dev. 2012; 88:503–7.

13. Costantine MM, Weiner SJ. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a metaanalysis. Obstet Gynecol. 2009; 114(2 pt 1):354–64.
14. Imamoglu EY, Gursoy T, Karatekin G, Ovali F. Effects of antenatal magnesium sulfate treatment on cerebral blood flow velocities in preterm neonates. J Perinatol. 2014; 34:192–6.

15. Hwang IT. The present condition of Korean children born small for gestational age. Korean J Pediatr. 2009; 52:137–41.

16. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982; 145:1–8.

17. Kim DH, Shim SY, Kim JR, Shin SH, Kim ES, Joung KE, et al. Recent outcome of extremely low birth weight infants: the use of CRIB (clinical risk index for babies) II score for analyzing the survival rate. Korean J Pediatr. 2006; 49:952–8.
19. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33:179–201.

20. An international classification of retinopathy of prematurity [editorial]. Pediatrics. 1984; 74:127–33.
21. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–34.

22. Su CY, Lin HC, Cheng HC, Yen AM, Chen YH, Kao S. Pregnancy outcomes of anti-hypertensives for women with chronic hypertension: a population-based study. PLoS ONE. 2013; 8:e53844.

23. Roberts CL, Algert CS, Morris JM, Ford JB, HendersonSmart DJ. Hypertensive disorders in pregnancy: a population-based study. Med J Aust. 2005; 182:332–5.

24. Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and metaanalysis. BMJ. 2014; 348:g2301.

25. Magee LA, Duley L. Oral beta-blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003; 3:CD002863.

26. Lennestal R, Otterblad Olausson P, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009; 65:615–25.

27. Hanssens M, Keirse MJ, Vankelecom F, Van Assche FA. Fetal and neonatal effects of treatment with angiotensinconverting enzyme inhibitors in pregnancy. Obstet Gynecol. 1991; 78:128–35.
28. Weitz C, Khouzami V, Maxwell K, Johnson JW. Treatment of hypertension in pregnancy with methyldopa: a randomized double blind study. Int J Gynaecol Obstet. 1987; 25:35–40.
29. Abalos E, Duley L, Steyn DW, HendersonSmart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007; 1:CD002252.

30. Han SP, Park SK, Song CH, Park J, Kim KS, Choi YY. The clinical and prognostic survey of the preterm infants delivered from pregnancy-induced hypertension mothers. J Korean Pediatr Soc. 2002; 45:64–71.
31. Kwon JH, Park JD, Kim BI, Choi JH, Yun CK. The prognosis of the newborn infants born to mothers of pregnancy induced hypertension. Korean J Perinatol. 1995; 6:142–50.
32. Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Magpie Trial Collaboration Group. Do women with preeclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebocontrolled trial. Lancet. 2002; 359:1877–90.
33. Kim CR. Effects of maternal hypertension on premature infants and neuroprotective role of magnesium. J Korean Pediatr Soc. 1997; 40:747–58.
34. Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002; 186:1111–8.

Go to : 
Table 1.
The Demographics and Clinical Characteristics
| HT (n = 150) | Control (n = 436) | P value | |
|---|---|---|---|
| Maternal characteristics | |||
| Male (%) | 84 (56.0) | 23 9(55.7) | 0.951 |
| Cesarean section (%) | 125 (83.3) | 279 (64.3) | 0.000 |
| Primipara (%) | 54 (36.0) | 142 (32.7) | 0.463 |
| Premature labor (%) | 65 (43.3) | 185 (42.8) | 0.953 |
| Antenatal steroid (%) | 90 (62.9) | 126 (29.0) | 0.000 |
| PROM (%) | 25 (16.6) | 167 (38.5) | 0.000 |
| Clinical chorioamnionitis (%) | 38 (25.3) | 73 (16.8) | 0.023 |
| Histological chorioamnionitis (%) | 16 (12.8) | 38 (11.6) | 0.730 |
| Neonatal demographics | |||
| Gestational age (wk)∗ | 32.2±3.5 | 33.7±3.0 | 0.000 |
| Birth weight (g)∗ | 1,810.5±689.2 | 2,212.1±604.9 | 0.000 |
| SGA (%)∗ | 22 (14.7) | 11 (2.5) | 0.000 |
| Apgar score at 1 minute∗ | 5.5±2.6 | 6.6±2.5 | 0.000 |
| Apgar score at 5 minute∗ | 7.2±2.1 | 8.1±2.2 | 0.000 |
| Length of stay (d)∗ | 31.0±29.3 | 17.3±19.3 | 0.000 |
| Duration of use of ventilation (d)∗ | 4.9±12.7 | 1.6±6.2 | 0.000 |
| Duration of oxygen supplement (d)∗ | 11.4±24.7 | 4.1 ±13.7 | 0.000 |
Table 2.
The Demographics and Clinical Characteristics between Treated Groups
| Anti-HT (n=40) | Anti-HT+Mg (n=110) | P value | |
|---|---|---|---|
| Maternal characteristics | |||
| Male (%) | 23 (57.5) | 61 (55.5) | 0.823 |
| Cesarean section (%) | 32 (80.0) | 93 (84.5) | 0.509 |
| Primipara (%) | 13 (32.5) | 41 (37.3) | 0.590 |
| Premature labor (%) | 16 (40.0) | 49 (44.5) | 0.619 |
| Antenatal steroid (%) | 16 (42.1) | 74 (70.5) | 0.002 |
| PROM (%) | 9 (22.5) | 16 (14.5) | 0.248 |
| Clinical chorioamnionitis (%) | 9 (22.5) | 29 (26.4) | 0.630 |
| Histological chorioamnionitis (%) | 3 (8.8) | 13 (14.3) | 0.416 |
| Neonatal demographics | |||
| Gestational age (week)∗ | 33.2±3.6 | 31.9±3.8 | 0.051 |
| Birth weight (g)∗ | 1,982.8±695.4 | 1,747.8±679.3 | 0.065 |
| SGA (%)∗ | 6 (15.0) | 16 (15.0) | 0.945 |
| Apgar score at 1 minute∗ | 6.3±2.6 | 5.3±2.5 | 0.031 |
| Apgar score at 5 minute∗ | 7.9±2.1 | 7.0±2.1 | 0.032 |
| Length of stay(d)∗ | 26.7±25.1 | 33.0±30.5 | 0.181 |
| Duration of use of ventilation (d)∗ | 2.7±6.5 (0–35, 0.625) | 5.7±14.2 (0–81, 0.682) | 0.078 |
| Duration of oxygen supplement (d)∗ | 7.9 ±18.4 (0–75, 0.414) | 12.9±26.8 (0–135, 1.375) | 0.205 |
Table 3.
Comparison of Neonatal Morbidities between Two Groups
Table 4.
Comparison of Neonatal Morbidities between Treated Groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download