Abstract
Parosteal lipoma is a rare benign tumor containing mature adipose tissue having an intimate relationship to the periosteum. Characteristically, this tumor presents as a lipomatous mass adjacent to bone, eliciting variable reactive changes in the underlying cortex. We report a case of parosteal lipoma of the foot. The MR findings consisted of juxtacortical lipomatous mass abutting to bony protuberance, with internal fibrous striations, and osseous reaction in the adjacent bone. By the aid of multiplanar imaging capability, high spatial and contrast resolution of MRI, characteristic features of parosteal lipoma can lead to diagnosis on imaging.
Parosteal lipoma is a rare benign fatty neoplasm containing mature adipose tissue that is firmly adherent to the periosteum of the underlying bone (1-5). The incidence of this tumor is 0.3% of all lipomas (2, 3, 6-11). Parosteal lipoma occurs almost exclusively in the extremities and is always solitary (3, 8-11). This tumor occurs most commonly in the 5th-7th decades and presents as a painless, non-tender slowly growing mass which is present for an average of 8-10 years (3, 8-11). To the best of our knowledge, there has been only one case report of the magnetic resonance (MR) imaging features of parosteal lipoma from Korea (12). We report a case of parosteal lipoma of the foot focusing on MR imaging findings.
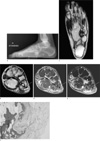
A 48-year-old woman presented with a progressive painful, palpable mass at the lateral aspect of her right foot, plantar surface. The patient didn't complain any symptoms related to nerve compression. Lateral radiograph of the right foot showed an irregular bony protruding lesion from the right 5th metatarsal base toward the plantar surface, with a surrounding well-circumscribed low-density soft tissue mass-like lesion (Fig. 1a). On non-enhanced coronal T1-weighted and axial T2-weighted MR images, a multi-lobulated and well-marginated high signal intensity mass with several thin low signal striations was noted in the plantar surface of right mid foot, between the 1st and 2nd muscle layers, just deep to the flexor digitorum brevis muscle (Fig. 1b, c). On fat-suppressed coronal T2-weighted images, almost whole area of the mass showed low signal intensity conversion representing fatty signal intensity with high signal thin striations (Fig. 1d). On enhanced fat-saturated coronal T1-weighted MR images, nearly most of the entire mass showed low signal intensity and minimal central thin septal enhancement was seen without a demonstrable enhancing solid portion, representing a benign lipomatous lesion (Fig. 1e). A focal nodular enhancement was seen in the junction between the mass and the bony protuberance, raising the possibility of reactive soft tissue change (arrowheads in Fig. 1b, d, e). A peripheral ill-defined subtle enhancement is seen in the soft tissue mass which corresponded to the high signal intensity portion on precontrast fat-suppressed T2-weighted images (thick arrows in Fig. 1b, d, e), representing secondary focal cystic change of the large lipomatous lesion. The mass showed irregular attachment to the cortical based bony excrescence arising from the inferior cortex of 5th metatarsal base (Fig. 1b-e). There was no definite medullary continuation between the bony protuberance and the 5th metatarsal bone on pre-contrast T1-weighted coronal images (Thin arrow in Fig. 1b). Although medullary continuation was suspected on the coronal fat-suppressed T2-weighted and post-contrast T1-weighted images, they are considered less reliable in the evaluation of the osseous medulla than T1-weighted images (thin arrows in Fig. 1b, d, e). The flexor digitorum brevis muscle was compressed, thinned, and was bulging inferiorly toward the plantar surface, without internal fatty striation suggesting atrophic change of the muscle (Fig. 1b, d, e). Focal external pressure erosion was combined in the inferior cortex of the 5th metatarsal base, medial to the bony excrescence, by a small lobulated portion of the soft tissue mass (curved arrows in Fig. 1b-e). The patient underwent complete mass excision including excision of the bony protuberance. At surgery, the lipomatous mass was yellowish, soft, and encapsulated, and strongly adherent to the underlying periosteum of the 5th metatarsal base and the bony protuberance, requiring subperiosteal dissection and the use of an osteotome for the removal of the soft tissue mass and the bony protuberance. The histologic diagnosis of the soft tissue mass was lipoma composed of mature adult fat (Fig. 1f). The junction between bony protuberance and the lipoma showed reactive cortical hypertrophic changes, cortical erosions, and fibrovascular proliferation of the intervening periosteum (Fig. 1f). There was no detectable cartilage or osteoid metaplasia adjacent to the osseous excrescence.
Lipoma is the most common benign tumor of the soft tissues and can be classified according to their location of origin, such as subcutaneous, intramuscular, intermuscular, intraosseous, intracortical, and parosteal. Parosteal lipoma is a rare benign fatty neoplasm containing mature adipose tissue that arises directly adjacent to bone, in continuity with the underlying periosteum (1-5). The incidence of this tumor is 0.3% of all lipomas (2, 3, 6-11). The most common sites of origin for parosteal lipomas are the thigh adjacent to the femur or the upper extremity near the proximal radius (3, 8-11). Other sites include the tibia, humerus, scapula, clavicle, ribs, pelvis, metacarpals, metatarsals, mandible, and skull. Patients with parosteal lipoma range from the 5th-7th decades and usually present with a history of a slowly growing, large, painless and nontender immobile mass not fixed to the skin (3, 8-11). Occasionally, compressive neuropathy may occur if the mass is sufficiently large or located in a strategic location that allows encroachment upon the nerve such as in the proximal radius (6, 7, 9).
Pathologically, parosteal lipomas are adherent to the underlying periosteum and are circumscribed greasy yellowish masses with thin, fibrous capsule. These lesions are composed of mature adult fat identical to soft-tissue lipomas. Cartilage, osteoid metaplasia, and areas of osseous excrescences or cortical thickening extending from and attaching the lesion to the bone surface are common (4, 13). These osseous excrescences do not show cortical or medullary continuity with the adjacent bone (2, 3). This relationship to the underlying bone distinguishes this lesion from a soft-tissue lipoma.
The radiographic features of parosteal lipoma are usually characteristic. In a study of parosteal lipoma by Fleming et al, nearly 70% of patients had abnormal underlying bone and 50% had osseous reaction. The most frequent osseous reactive changes included bowing of bone or cortical erosion secondary to the adjacent lipomatous mass. In a study by Murphey et al (2), the major radiographic features of parosteal lipoma included a juxtacortical radiolucent lipomatous mass with varying degrees of septation associated with surface bone productive changes ranging from very subtle to obvious cortical thickening and variably sized ossific protuberances or excrescences. These areas of cortical abnormality do not show medullary continuity with the underlying bone, as would be expected in an osteochondroma (2, 3).
On MR images, the tumor is identified as a juxtacortical mass with signal intensity identical to that of subcutaneous fat, regardless of pulse sequence (2). Heterogeneity in these lesions is invariably present and corresponds to the pathologic components in the lesion. Areas with intermediate signal intensity on T1-weighted images that are high signal intensity on T2-weighted images represent the cartilaginous components in parosteal lipoma (2). Fibrovascular septa may cause a lobulated appearance of the fat component, with low-signal-intensity strands on T1-weighted images that become higher in signal intensity on the long TR images (particularly with fat suppression). These thin fibrous septa are different from those of well-differentiated liposarcoma, which are thick and show marked enhancement (14). Larger areas of bone production surrounded by the lipomatous components are also well demonstrated with MR imaging. Adjacent muscle atrophy, poorly demonstrated by CT, is identified on MR images as increased striations of fat in the affected muscle and is caused by associated nerve entrapment(2). This finding is best appreciated on T2-weighted images because of the decreased signal intensity of normal muscle relative to fat. In contrast with radiography and CT imaging, which highlights the bony protuberance and changes of underlying bone, MR imaging best demonstrates the soft tissue portion and relationship of the tumor to the underlying native bone and muscle, and this information is important for surgical planning because parosteal lipoma is usually firmly adherent to the underlying cortex at the site of surface bone production.
In our case, bony protuberance and surrounding juxtacortical benign lipoma was seen in the plantar surface of the right 5th metatarsal base, with focal cortical erosion, suggestive of the typical findings of parosteal lipoma, which were well appreciated in MR imaging. The exclusion of medullary continuity between bony protuberance and the adjacent bone, which is the differential diagnostic clue from osteochondroma, was also well appreciated in the precontrast T1-weighted images. Additionally, no findings related with nerve compression were detected in MR imaging, providing confidence to the clinical examination results.
In summary, by the aid of imaging capability, high spatial and contrast resolution of MRI, characteristic features of parosteal lipoma can lead to a reliable diagnosis on imaging. Recognition of these characteristic features is important because of the apparent lack of malignant potential of parosteal lipoma.
Figures and Tables
Fig. 1
A 48-year-old female patient with parosteal lipoma in the right foot.
(a) Lateral radiograph of the right foot shows an irregular bony protrusion from right 5th metatarsal base with surrounding low-density soft tissue mass-like lesion (arrows). Non-enhanced coronal T1-weighted (b), axial T2-weighted (c) MR images show a multi-lobulated well-marginated high signal intensity mass (asterisks) with several thin low signal striations(open arrows) in the plantar surface of right mid foot just deep to the flexor digitorum brevis muscle. (d) Fat-suppressed coronal T2-weighted image shows low signal intensity conversion in almost whole area of the mass (asterisks) and high signal thin striations (open arrow). (e) Post-contrast fat-saturated coronal T1-weighted image shows minimal enhancement in the internal striations (open arrow) without demonstrable enhancing solid portion in the mass. A focal nodular enhancement is seen in the junction between the bony protuberance and the mass (arrowheads). A peripheral ill-defined subtle enhancement is seen in the mass which corresponds to the high signal portion on pre-contrast fat-suppressed T2-weighted image (thick arrows in Fig. b, d, e), representing secondary focal cystic change. The mass shows irregular attachment (arrowheads in Fig. b, d, e) to the cortical based bony excrescence arising from the inferior aspect of the 5th metatarsal base (B on Fig. b-e). Medullary continuation between bony protuberance and the 5th metatarsal bone is not definite on the precontrast T1-weighted coronal image, although suspected in the other pulse sequences (thin arrows in Fig. b, d, e). Focal cortical erosion is combined in the inferomedial side of the 5th metatarsal base (curved arrows in Fig. b-e). (f) Photomicrograph (×40, H-E stain) reveals the junction between bony protuberance (B) and the lipoma (L) with reactive cortical hypertrophic changes, cortical erosions (arrow), and fibrovascular proliferation of the intervening periosteum (P).
References
1. Kransdorf MJ, Moser RP Jr, Meis JM, Meyer CA. Fat-containing soft-tissue masses of the extremities. Radiographics. 1991. 11:81–106.
2. Murphey MD, Johnson DL, Bhatia PS, Neff JR, Rosenthal HG, Walker CW. Parosteal lipoma: MR imaging characteristics. AJR Am J Roentgenol. 1994. 162:105–110.
3. Ramos A, Castello J, Sartoris DJ, Greenway GD, Resnick D, Haghighi P. Osseous lipoma: CT appearance. Radiology. 1985. 157:615–619.
4. Jones JG, Habermann ET, Dorfman HD. Case report 553. Parosteal ossifying lipoma of femur. Skeletal Radiol. 1989. 18:537–540.
5. Kawashima A, Magid D, Fishman EK, Hruban RH, Ney DR. Parosteal ossifying lipoma: CT and MR findings. J Comput Assist Tomogr. 1993. 17:147–150.
6. Nishida J, Shimamura T, Ehara S, Shiraishi H, Sato T, Abe M. Posterior interosseous nerve palsy caused by parosteal lipoma of proximal radius. Skeletal Radiol. 1998. 27:375–379.
7. Henrique A. A high radial neuropathy by parosteal lipoma compression. J Shoulder Elbow Surg. 2002. 11:386–388.
8. Jacobs P. Parosteal lipoma with hyperostosis. Clin Radiol. 1972. 23:196–198.
9. Moon N, Marmor L. Parostel Lipoma of the Proximal Part of the Radius. A Clinical Entity with Frequent Radial-Nerve Injury. J Bone Joint Surg Am. 1964. 46:608–614.
10. Krajewska I, Vernon-Roberts B, Sorby-Adams G. Parosteal (periosteal) lipoma. Pathology. 1988. 20:179–183.
11. Berry JB, Moiel RH. Parosteal lipoma producing paralysis of the deep radial nerve. South Med J. 1973. 66:1298–1300.
12. Yu JS, Weis L, Becker W. MR imaging of a parosteal lipoma. Clin Imaging. 2000. 24:15–18.
13. Demos TC, Bruno E, Armin A, Dobozi WR. Parosteal lipoma with enlarging osteochondroma. AJR Am J Roentgenol. 1984. 143:365–366.
14. Ohguri T, Aoki T, Hisaoka M, Watanabe H, Nakamura K, Hashimoto H, et al. Differential diagnosis of benign peripheral lipoma from well-differentiated liposarcoma on MR imaging: is comparison of margins and internal characteristics useful? AJR Am J Roentgenol. 2003. 180:1689–1694.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download