3. Polymeropoulos MH, Lavedan C, Leroy E, et al. 1997; Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 276:2045–2047. DOI:
10.1126/science.276.5321.2045. PMID:
9197268.
4. Singleton AB, Farrer M, Johnson J, et al. 2003; α-Synuclein locus triplication causes Parkinson's disease. Science. 302:841. DOI:
10.1126/science.1090278. PMID:
14593171.
5. Lee VM, Trojanowski JQ. 2006; Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 52:33–38. DOI:
10.1016/j.neuron.2006.09.026. PMID:
17015225.
7. Basellini MJ, Kothuis JM, Comincini A, Pezzoli G, Cappelletti G, Mazzetti S. 2023; Pathological pathways and alpha-synuclein in Parkinson's disease: a view from the periphery. Front Biosci (Landmark Ed). 28:33. DOI:
10.31083/j.fbl2802033. PMID:
36866559.
8. Golpich M, Amini E, Mohamed Z, Azman Ali R, Mohamed Ibrahim N, Ahmadiani A. 2017; Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther. 23:5–22. DOI:
10.1111/cns.12655. PMID:
27873462. PMCID:
PMC6492703.
10. Schapira AH. 2007; Mitochondrial dysfunction in Parkinson's disease. Cell Death Differ. 14:1261–1266. DOI:
10.1038/sj.cdd.4402160. PMID:
17464321.
12. Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, et al. 2011; Distinct roles
in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J Neurosci. 31:14508–14520. DOI:
10.1523/JNEUROSCI.1560-11.2011. PMID:
21994367. PMCID:
PMC3587176.
13. Kulkarni A, Preeti K, Tryphena KP, Srivastava S, Singh SB, Khatri DK. 2023; Proteostasis in Parkinson's disease: recent development and possible implication in diagnosis and therapeutics. Ageing Res Rev. 84:101816. DOI:
10.1016/j.arr.2022.101816. PMID:
36481490.
15. Srinivasan E, Chandrasekhar G, Chandrasekar P, et al. 2021; Alpha-synuclein aggregation in Parkinson's disease. Front Med (Lausanne). 8:736978. DOI:
10.3389/fmed.2021.736978. PMID:
34733860. PMCID:
PMC8558257.
16. Chu YT, Tai CH, Lin CH, Wu RM. 2021; Updates on the genetics of Parkinson's disease: clinical implications and future treatment. Acta Neurol Taiwan. 30:83–93.
17. Connolly BS, Lang AE. 2014; Pharmacological treatment of Parkinson disease: a review. JAMA. 311:1670–1683. DOI:
10.1001/jama.2014.3654. PMID:
24756517.
18. Olanow CW, Stern MB, Sethi K. 2009; The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 72(21 Suppl 4):S1–S136. DOI:
10.1212/WNL.0b013e3181a1d44c.
19. Cheong SL, Federico S, Spalluto G, Klotz KN, Pastorin G. 2019; The current status of pharmacotherapy for the treatment of Parkinson's disease: transition from single-target to multitarget therapy. Drug Discov Today. 24:1769–1783. DOI:
10.1016/j.drudis.2019.05.003. PMID:
31102728.
20. LeWitt PA, Fahn S. 2016; Levodopa therapy for Parkinson disease: a look backward and forward. Neurology. 86(14 Suppl 1):S3–S12. DOI:
10.1212/WNL.0000000000002509. PMID:
27044648.
21. Stocchi F, Tagliati M, Olanow CW. 2008; Treatment of levodopa-induced motor complications. Mov Disord. 23 Suppl 3:S599–S612. DOI:
10.1002/mds.22052. PMID:
18781681.
22. Parkinson Study Group. 2004; A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch Neurol. 61:561–566. DOI:
10.1001/archneur.61.4.561. PMID:
15096406.
23. Siddiqi SH, Abraham NK, Geiger CL, Karimi M, Perlmutter JS, Black KJ. 2016; The human experience with intravenous levodopa. Front Pharmacol. 6:307. DOI:
10.3389/fphar.2015.00307. PMID:
26779024. PMCID:
PMC4701937.
24. Freckelton I. 2022; Parkinson's disease and the criminal justice system. J Law Med. 29:309–321.
27. Sivandzade F, Cucullo L. 2021; Regenerative stem cell therapy for neurodegenerative diseases: an overview. Int J Mol Sci. 22:2153. DOI:
10.3390/ijms22042153. PMID:
33671500. PMCID:
PMC7926761.
28. Liu G, David BT, Trawczynski M, Fessler RG. 2020; Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev Rep. 16:3–32. DOI:
10.1007/s12015-019-09935-x. PMID:
31760627. PMCID:
PMC6987053.
29. Barbuti PA, Barker RA, Brundin P, et al. MDS Scientific Issues Committee. 2021; Recent advances in the development of stem-cell-derived dopaminergic neuronal transplant therapies for Parkinson's disease. Mov Disord. 36:1772–1780. DOI:
10.1002/mds.28628. PMID:
33963552.
30. Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. 2002; The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 22:6639–6649. DOI:
10.1523/JNEUROSCI.22-15-06639.2002. PMID:
12151543. PMCID:
PMC6758128.
31. Cha Y, Park TY, Leblanc P, Kim KS. 2023; Current status and future perspectives on stem cell-based therapies for Parkinson's disease. J Mov Disord. 16:22–41. DOI:
10.14802/jmd.22141. PMID:
36628428. PMCID:
PMC9978267.
32. Palmer TD, Takahashi J, Gage FH. 1997; The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 8:389–404. DOI:
10.1006/mcne.1996.0595. PMID:
9143557.
33. Kempermann G, Wiskott L, Gage FH. 2004; Functional significance of adult neurogenesis. Curr Opin Neurobiol. 14:186–191. DOI:
10.1016/j.conb.2004.03.001. PMID:
15082323.
34. Fallon J, Reid S, Kinyamu R, et al. 2000;
In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 97:14686–14691. DOI:
10.1073/pnas.97.26.14686. PMID:
11121069. PMCID:
PMC18979.
35. Zhao M, Momma S, Delfani K, et al. 2003; Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 100:7925–7930. DOI:
10.1073/pnas.1131955100. PMID:
12792021. PMCID:
PMC164689.
36. Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. 2001; Cell culture. Progenitor cells from human brain after death. Nature. 411:42–43. DOI:
10.1038/35075141. PMID:
11333968.
37. Parmar M, Skogh C, Englund U. 2003; A transplantation study of expanded human embryonic forebrain precursors: evidence for selection of a specific progenitor population. Mol Cell Neurosci. 23:531–543. DOI:
10.1016/S1044-7431(03)00097-6. PMID:
12932435.
38. De Gioia R, Biella F, Citterio G, et al. 2020; Neural stem cell transplantation for neurodegenerative diseases. Int J Mol Sci. 21:3103. DOI:
10.3390/ijms21093103. PMID:
32354178. PMCID:
PMC7247151.
39. Björklund A, Dunnett SB, Brundin P, et al. 2003; Neural transplantation for the treatment of Parkinson's disease. Lancet Neurol. 2:437–445. DOI:
10.1016/S1474-4422(03)00442-3. PMID:
12849125.
40. Andersson EK, Irvin DK, Ahlsiö J, Parmar M. 2007; Ngn2 and Nurr1 act in synergy to induce midbrain dopaminergic neurons from expanded neural stem and progenitor cells. Exp Cell Res. 313:1172–1180. DOI:
10.1016/j.yexcr.2006.12.014. PMID:
17291494.
42. Ganat YM, Calder EL, Kriks S, et al. 2012; Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 122:2928–2939. DOI:
10.1172/JCI58767. PMID:
22751106. PMCID:
PMC3408729.
45. Kriks S, Shim JW, Piao J, et al. 2011; Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 480:547–551. DOI:
10.1038/nature10648. PMID:
22056989. PMCID:
PMC3245796.
46. Kim TW, Piao J, Koo SY, et al. 2021; Biphasic activation of WNT signaling facilitates the derivation of midbrain dopamine neurons from hESCs for translational use. Cell Stem Cell. 28:343–355.e5. DOI:
10.1016/j.stem.2021.01.005. PMID:
33545081. PMCID:
PMC8006469.
47. Schweitzer JS, Song B, Herrington TM, et al. 2020; Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease. N Engl J Med. 382:1926–1932. DOI:
10.1056/NEJMoa1915872. PMID:
32402162. PMCID:
PMC7288982.
48. Park TY, Jeon J, Lee N, et al. 2023; Co-transplantation of autologous Treg cells in a cell therapy for Parkinson's disease. Nature. 619:606–615. DOI:
10.1038/s41586-023-06300-4. PMID:
37438521.
49. Wang YK, Zhu WW, Wu MH, et al. 2018; Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson's disease. Stem Cell Reports. 11:171–182. DOI:
10.1016/j.stemcr.2018.05.010. PMID:
29910127. PMCID:
PMC6067059.
50. Piao J, Zabierowski S, Dubose BN, et al. 2021; Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell. 28:217–229.e7. DOI:
10.1016/j.stem.2021.01.004. PMID:
33545080. PMCID:
PMC7903922.
51. Kirkeby A, Parmar M, Barker RA. 2017; Strategies for bringing stem cell-derived dopamine neurons to the clinic: a European approach (STEM-PD). Prog Brain Res. 230:165–190. DOI:
10.1016/bs.pbr.2016.11.011. PMID:
28552228.
53. Kirkeby A, Nelander J, Hoban DB, et al. 2023; Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson's disease, STEM-PD. Cell Stem Cell. 30:1299–1314.e9. DOI:
10.1016/j.stem.2023.08.014. PMID:
37802036.
54. Hauser RA, Freeman TB, Snow BJ, et al. 1999; Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol. 56:179–187. DOI:
10.1001/archneur.56.2.179. PMID:
10025423.
55. Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. 2006; Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 12:1259–1268. DOI:
10.1038/nm1495. PMID:
17057709.
56. Redmond DE Jr, Bjugstad KB, Teng YD, et al. 2007; Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci U S A. 104:12175–12180. DOI:
10.1073/pnas.0704091104. PMID:
17586681. PMCID:
PMC1896134.
57. Bachoud-Lévi AC, Gaura V, Brugières P, et al. 2006; Effect of fetal neural transplants in patients with Huntington's disease 6 years after surgery: a long-term follow-up study. Lancet Neurol. 5:303–309. DOI:
10.1016/S1474-4422(06)70381-7. PMID:
16545746.
58. Mendez I, Viñuela A, Astradsson A, et al. 2008; Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 14:507–509. DOI:
10.1038/nm1752. PMID:
18391961. PMCID:
PMC2656682.
59. Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. 2008; Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 14:504–506. DOI:
10.1038/nm1747. PMID:
18391962.
60. Li JY, Englund E, Holton JL, et al. 2008; Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 14:501–503. DOI:
10.1038/nm1746. PMID:
18391963.
61. Barker RA, Drouin-Ouellet J, Parmar M. 2015; Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol. 11:492–503. DOI:
10.1038/nrneurol.2015.123. PMID:
26240036.
62. Kikuchi T, Morizane A, Doi D, et al. 2017; Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 548:592–596. DOI:
10.1038/nature23664. PMID:
28858313.
63. Kyttälä A, Moraghebi R, Valensisi C, et al. 2016; Genetic variability overrides the impact of parental cell type and determines iPSC differentiation potential. Stem Cell Reports. 6:200–212. DOI:
10.1016/j.stemcr.2015.12.009. PMID:
26777058. PMCID:
PMC4750096.
64. Singh VK, Kalsan M, Kumar N, Saini A, Chandra R. 2015; Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 3:2. DOI:
10.3389/fcell.2015.00002. PMID:
25699255. PMCID:
PMC4313779.
65. Allan LE, Petit GH, Brundin P. 2010; Cell transplantation in Parkinson's disease: problems and perspectives. Curr Opin Neurol. 23:426–432. DOI:
10.1097/WCO.0b013e32833b1f62. PMID:
20489615.
66. Kim TW, Koo SY, Studer L. 2020; Pluripotent stem cell therapies for Parkinson disease: present challenges and future opportunities. Front Cell Dev Biol. 8:729. DOI:
10.3389/fcell.2020.00729. PMID:
32903681. PMCID:
PMC7438741.
68. Nishimura T, Xu H, Iwasaki M, et al. 2019; Sufficiency for inducible Caspase-9 safety switch in human pluripotent stem cells and disease cells. Gene Ther. 27:525–534. DOI:
10.1038/s41434-020-0179-z. PMID:
32704085.
69. Katsukawa M, Nakajima Y, Fukumoto A, Doi D, Takahashi J. 2016; Fail-safe therapy by gamma-ray irradiation against tumor formation by human-induced pluripotent stem cell-derived neural progenitors. Stem Cells Dev. 25:815–825. DOI:
10.1089/scd.2015.0394. PMID:
27059007.
70. Takagi Y, Takahashi J, Saiki H, et al. 2005; Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 115:102–109. DOI:
10.1172/JCI21137. PMID:
15630449. PMCID:
PMC539189.
72. Parmar M. 2018; Towards stem cell based therapies for Parkinson's disease. Development. 145:dev156117. DOI:
10.1242/dev.156117. PMID:
29311261.
73. Volarevic V, Markovic BS, Gazdic M, et al. 2018; Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 15:36–45. DOI:
10.7150/ijms.21666. PMID:
29333086. PMCID:
PMC5765738.
75. Lovell-Badge R, Anthony E, Barker RA, et al. 2021; ISSCR guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Reports. 16:1398–1408. DOI:
10.1016/j.stemcr.2021.05.012. PMID:
34048692. PMCID:
PMC8190668.
76. Sullivan S, Stacey GN, Akazawa C, et al. 2018; Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen Med. 13:859–866. DOI:
10.2217/rme-2018-0095. PMID:
30205750.
77. Lee G, Papapetrou EP, Kim H, et al. 2009; Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 461:402–406. DOI:
10.1038/nature08320. PMID:
19693009. PMCID:
PMC2784695.
78. Mukherjee-Clavin B, Mi R, Kern B, et al. 2019; Comparison of three congruent patient-specific cell types for the modelling of a human genetic Schwann-cell disorder. Nat Biomed Eng. 3:571–582. DOI:
10.1038/s41551-019-0381-8. PMID:
30962586. PMCID:
PMC6612317.
79. Kim YJ, Lim H, Li Z, et al. 2014; Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell. 15:497–506. DOI:
10.1016/j.stem.2014.07.013. PMID:
25158936.
80. Lee G, Ramirez CN, Kim H, et al. 2012; Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol. 30:1244–1248. DOI:
10.1038/nbt.2435. PMID:
23159879. PMCID:
PMC3711177.
81. Choi IY, Lim HT, Che YH, Lee G, Kim YJ. 2021; Inhibition of the combinatorial signaling of transforming growth factor-beta and NOTCH promotes myotube formation of human pluripotent stem cell-derived skeletal muscle progenitor cells. Cells. 10:1649. DOI:
10.3390/cells10071649. PMID:
34209364. PMCID:
PMC8303216.
82. Lee G, Studer L. 2011; Modelling familial dysautonomia in human induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci. 366:2286–2296. DOI:
10.1098/rstb.2011.0026. PMID:
21727134. PMCID:
PMC3130420.
83. Cooper O, Seo H, Andrabi S, et al. 2012; Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson's disease. Sci Transl Med. 4:141ra90. DOI:
10.1126/scitranslmed.3003985.
84. Byers B, Cord B, Nguyen HN, et al. 2011; SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate α-synuclein and are susceptible to oxidative stress. PLoS One. 6:e26159. DOI:
10.1371/journal.pone.0026159. PMID:
22110584. PMCID:
PMC3217921.
86. Devine MJ, Ryten M, Vodicka P, et al. 2011; Parkinson's disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2:440. DOI:
10.1038/ncomms1453. PMID:
21863007. PMCID:
PMC3265381.
87. Heman-Ackah SM, Manzano R, Hoozemans JJM, et al. 2017; Alpha-synuclein induces the unfolded protein response in Parkinson's disease SNCA triplication iPSC-derived neurons. Hum Mol Genet. 26:4441–4450. DOI:
10.1093/hmg/ddx331. PMID:
28973645. PMCID:
PMC5886237.
88. Stojkovska I, Wani WY, Zunke F, et al. 2022; Rescue of α-synuclein aggregation in Parkinson's patient neurons by synergistic enhancement of ER proteostasis and protein trafficking. Neuron. 110:436–451.e11. DOI:
10.1016/j.neuron.2021.10.032. PMID:
34793693. PMCID:
PMC8815333.
89. Prots I, Grosch J, Brazdis RM, et al. 2018; α-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc Natl Acad Sci U S A. 115:7813–7818. DOI:
10.1073/pnas.1713129115. PMID:
29991596. PMCID:
PMC6065020.
90. Ludtmann MHR, Angelova PR, Horrocks MH, et al. 2018; α-Synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson's disease. Nat Commun. 9:2293. DOI:
10.1038/s41467-018-04422-2. PMID:
29895861. PMCID:
PMC5997668.
91. Diao X, Wang F, Becerra-Calixto A, Soto C, Mukherjee A. 2021; Induced pluripotent stem cell-derived dopaminergic neurons from familial Parkinson's disease patients display α-synuclein pathology and abnormal mitochondrial morphology. Cells. 10:2402. DOI:
10.3390/cells10092402. PMID:
34572052. PMCID:
PMC8467069.
92. Zambon F, Cherubini M, Fernandes HJR, et al. 2019; Cellular α-synuclein pathology is associated with bioenergetic dysfunction in Parkinson's iPSC-derived dopamine neurons. Hum Mol Genet. 28:2001–2013. DOI:
10.1093/hmg/ddz038. PMID:
30753527. PMCID:
PMC6548224.
93. Brazdis RM, Alecu JE, Marsch D, et al. 2020; Demonstration of brain region-specific neuronal vulnerability in human iPSC-based model of familial Parkinson's disease. Hum Mol Genet. 29:1180–1191. DOI:
10.1093/hmg/ddaa039. PMID:
32160287. PMCID:
PMC7206857.
94. Chung CY, Khurana V, Auluck PK, et al. 2013; Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science. 342:983–987. DOI:
10.1126/science.1245296. PMID:
24158904. PMCID:
PMC4022187.
95. Ryan SD, Dolatabadi N, Chan SF, et al. 2013; Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell. 155:1351–1364. DOI:
10.1016/j.cell.2013.11.009. PMID:
24290359. PMCID:
PMC4028128.
96. Czaniecki C, Ryan T, Stykel MG, et al. 2019; Axonal pathology in hPSC-based models of Parkinson's disease results from loss of Nrf2 transcriptional activity at the Map1b gene locus. Proc Natl Acad Sci U S A. 116:14280–14289. DOI:
10.1073/pnas.1900576116. PMID:
31235589. PMCID:
PMC6628831.
98. Aflaki E, Borger DK, Moaven N, et al. 2016; A new glucocerebrosidase chaperone reduces α-synuclein and glycolipid levels in iPSC-derived dopaminergic neurons from patients with Gaucher disease and Parkinsonism. J Neurosci. 36:7441–7452. DOI:
10.1523/JNEUROSCI.0636-16.2016. PMID:
27413154. PMCID:
PMC4945664.
99. Woodard CM, Campos BA, Kuo SH, et al. 2014; iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson's disease. Cell Rep. 9:1173–1182. DOI:
10.1016/j.celrep.2014.10.023. PMID:
25456120. PMCID:
PMC4255586.
100. Fernandes HJ, Hartfield EM, Christian HC, et al. 2016; ER stress and autophagic perturbations lead to elevated extracellular α-synuclein in GBA-N370S Parkinson's iPSC-derived dopamine neurons. Stem Cell Reports. 6:342–356. DOI:
10.1016/j.stemcr.2016.01.013. PMID:
26905200. PMCID:
PMC4788783.
101. Schöndorf DC, Ivanyuk D, Baden P, et al. 2018; The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson's disease. Cell Rep. 23:2976–2988. DOI:
10.1016/j.celrep.2018.05.009. PMID:
29874584.
102. Aboud AA, Tidball AM, Kumar KK, et al. 2012; Genetic risk for Parkinson's disease correlates with alterations in neuronal manganese sensitivity between two human subjects. Neurotoxicology. 33:1443–1449. DOI:
10.1016/j.neuro.2012.10.009. PMID:
23099318. PMCID:
PMC3518601.
103. Jiang H, Ren Y, Yuen EY, et al. 2012; Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun. 3:668. DOI:
10.1038/ncomms1669. PMID:
22314364. PMCID:
PMC3498452.
104. Chung SY, Kishinevsky S, Mazzulli JR, et al. 2016; Parkin and PINK1 patient iPSC-derived midbrain dopamine neurons exhibit mitochondrial dysfunction and α-synuclein accumulation. Stem Cell Reports. 7:664–677. DOI:
10.1016/j.stemcr.2016.08.012. PMID:
27641647. PMCID:
PMC5063469.
105. Oh CK, Sultan A, Platzer J, et al. 2017; S-nitrosylation of PINK1 attenuates PINK1/Parkin-dependent mitophagy in hiPSC-based Parkinson's disease models. Cell Rep. 21:2171–2182. DOI:
10.1016/j.celrep.2017.10.068. PMID:
29166608. PMCID:
PMC5705204.
106. Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D. 2011; Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 31:5970–5976. DOI:
10.1523/JNEUROSCI.4441-10.2011. PMID:
21508222. PMCID:
PMC3091622.
107. Nguyen HN, Byers B, Cord B, et al. 2011; LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 8:267–280. DOI:
10.1016/j.stem.2011.01.013. PMID:
21362567. PMCID:
PMC3578553.
108. Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, et al. 2012; Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol Med. 4:380–395. DOI:
10.1002/emmm.201200215. PMID:
22407749. PMCID:
PMC3403296.
109. Boecker CA, Goldsmith J, Dou D, Cajka GG, Holzbaur ELF. 2021; Increased LRRK2 kinase activity alters neuronal autophagy by disrupting the axonal transport of autophagosomes. Curr Biol. 31:2140–2154.e6. DOI:
10.1016/j.cub.2021.02.061. PMID:
33765413. PMCID:
PMC8154747.
110. Hsieh CH, Shaltouki A, Gonzalez AE, et al. 2016; Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson's disease. Cell Stem Cell. 19:709–724. DOI:
10.1016/j.stem.2016.08.002. PMID:
27618216. PMCID:
PMC5135570.
111. Orenstein SJ, Kuo SH, Tasset I, et al. 2013; Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci. 16:394–406. DOI:
10.1038/nn.3350. PMID:
23455607. PMCID:
PMC3609872.
112. Sanders LH, Laganière J, Cooper O, et al. 2014; LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: reversal by gene correction. Neurobiol Dis. 62:381–386. DOI:
10.1016/j.nbd.2013.10.013. PMID:
24148854. PMCID:
PMC3877733.
113. Sheng ZH, Cai Q. 2012; Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 13:77–93. DOI:
10.1038/nrn3156. PMID:
22218207. PMCID:
PMC4962561.
114. Schwab AJ, Sison SL, Meade MR, Broniowska KA, Corbett JA, Ebert AD. 2017; Decreased sirtuin deacetylase activity in LRRK2 G2019S iPSC-derived dopaminergic neurons. Stem Cell Reports. 9:1839–1852. DOI:
10.1016/j.stemcr.2017.10.010. PMID:
29129681. PMCID:
PMC5785678.
115. Korecka JA, Talbot S, Osborn TM, et al. 2019; Neurite collapse and altered ER Ca2
+ control in human Parkinson disease patient iPSC-derived neurons with LRRK2 G2019S mutation. Stem Cell Reports. 12:29–41. DOI:
10.1016/j.stemcr.2018.11.021. PMID:
30595548. PMCID:
PMC6335600.
116. Gonzalez-Cano L, Menzl I, Tisserand J, Nicklas S, Schwamborn JC. 2018; Parkinson's disease-associated mutant LRRK2-mediated inhibition of miRNA activity is antagonized by TRIM32. Mol Neurobiol. 55:3490–3498. DOI:
10.1007/s12035-017-0570-y. PMID:
28508149. PMCID:
PMC5842508.
117. Bono K, Hara-Miyauchi C, Sumi S, Oka H, Iguchi Y, Okano HJ. 2020; Endosomal dysfunction in iPSC-derived neural cells from Parkinson's disease patients with VPS35 D620N. Mol Brain. 13:137. DOI:
10.1186/s13041-020-00675-5. PMID:
33032646. PMCID:
PMC7542911.
118. Cookson MR. 2010; The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nat Rev Neurosci. 11:791–797. DOI:
10.1038/nrn2935. PMID:
21088684. PMCID:
PMC4662256.
119. Liu GH, Qu J, Suzuki K, et al. 2012; Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature. 491:603–607. DOI:
10.1038/nature11557. PMID:
23075850. PMCID:
PMC3504651.
120. Reinhardt P, Schmid B, Burbulla LF, et al. 2013; Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 12:354–367. DOI:
10.1016/j.stem.2013.01.008. PMID:
23472874.
121. Tabata Y, Imaizumi Y, Sugawara M, et al. 2018; T-type calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson disease. Stem Cell Reports. 11:1171–1184. DOI:
10.1016/j.stemcr.2018.09.006. PMID:
30344006. PMCID:
PMC6234903.
122. Burbulla LF, Jeon S, Zheng J, Song P, Silverman RB, Krainc D. 2019; A modulator of wild-type glucocerebrosidase improves pathogenic phenotypes in dopaminergic neuronal models of Parkinson's disease. Sci Transl Med. 11:eaau6870. DOI:
10.1126/scitranslmed.aau6870. PMID:
31619543. PMCID:
PMC7359409.
124. Munsie LN, Milnerwood AJ, Seibler P, et al. 2015; Retromer-dependent neurotransmitter receptor trafficking to synapses is altered by the Parkinson's disease VPS35 mutation p.D620N. Hum Mol Genet. 24:1691–1703. DOI:
10.1093/hmg/ddu582. PMID:
25416282.
125. Hirano K, Fujimaki M, Sasazawa Y, et al. 2019; Neuroprotective effects of memantine via enhancement of autophagy. Biochem Biophys Res Commun. 518:161–170. DOI:
10.1016/j.bbrc.2019.08.025. PMID:
31431260.
126. Fernández-Santiago R, Carballo-Carbajal I, Castellano G, et al. 2015; Aberrant epigenome in iPSC-derived dopaminergic neurons from Parkinson's disease patients. EMBO Mol Med. 7:1529–1546. DOI:
10.15252/emmm.201505439. PMID:
26516212. PMCID:
PMC4693505.
127. Laperle AH, Sances S, Yucer N, et al. 2020; iPSC modeling of young-onset Parkinson's disease reveals a molecular signature of disease and novel therapeutic candidates. Nat Med. 26:289–299. DOI:
10.1038/s41591-019-0739-1. PMID:
31988461.
128. Burbulla LF, Song P, Mazzulli JR, et al. 2017; Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science. 357:1255–1261. DOI:
10.1126/science.aam9080. PMID:
28882997. PMCID:
PMC6021018.
129. Kouroupi G, Taoufik E, Vlachos IS, et al. 2017; Defective synaptic connectivity and axonal neuropathology in a human iPSC-based model of familial Parkinson's disease. Proc Natl Acad Sci U S A. 114:E3679–E3688. DOI:
10.1073/pnas.1617259114. PMID:
28416701. PMCID:
PMC5422768.
130. Fanning S, Haque A, Imberdis T, et al. 2019; Lipidomic analysis of α-synuclein neurotoxicity identifies stearoyl CoA desaturase as a target for Parkinson treatment. Mol Cell. 73:1001–1014.e8. DOI:
10.1016/j.molcel.2018.11.028. PMID:
30527540. PMCID:
PMC6408259.
131. Gandelman M, Dansithong W, Kales SC, et al. 2021; The AKT modulator A-443654 reduces α-synuclein expression and normalizes ER stress and autophagy. J Biol Chem. 297:101191. DOI:
10.1016/j.jbc.2021.101191. PMID:
34520759. PMCID:
PMC8482485.
132. Ho GPH, Ramalingam N, Imberdis T, Wilkie EC, Dettmer U, Selkoe DJ. 2021; Upregulation of cellular palmitoylation mitigates α-synuclein accumulation and neurotoxicity. Mov Disord. 36:348–359. DOI:
10.1002/mds.28346. PMID:
33103814. PMCID:
PMC8887921.
133. Lin M, Mackie PM, Shaerzadeh F, et al. 2021; In Parkinson's patient-derived dopamine neurons, the triplication of α-synuclein locus induces distinctive firing pattern by impeding D2 receptor autoinhibition. Acta Neuropathol Commun. 9:107. DOI:
10.1186/s40478-021-01203-9. PMID:
34099060. PMCID:
PMC8185945.
134. Mazzulli JR, Zunke F, Tsunemi T, et al. 2016; Activation of β-glucocerebrosidase reduces pathological α-synuclein and restores lysosomal function in Parkinson's patient midbrain neurons. J Neurosci. 36:7693–7706. DOI:
10.1523/JNEUROSCI.0628-16.2016. PMID:
27445146. PMCID:
PMC4951575.
135. Burbulla LF, Zheng J, Song P, et al. 2021; Direct targeting of wild-type glucocerebrosidase by antipsychotic quetiapine improves pathogenic phenotypes in Parkinson's disease models. JCI Insight. 6:e148649. DOI:
10.1172/jci.insight.148649. PMID:
34622801. PMCID:
PMC8525588.
136. Ren Y, Jiang H, Hu Z, et al. 2015; Parkin mutations reduce the complexity of neuronal processes in iPSC-derived human neurons. Stem Cells. 33:68–78. DOI:
10.1002/stem.1854. PMID:
25332110. PMCID:
PMC4429885.
137. Yamaguchi A, Ishikawa KI, Inoshita T, et al. 2020; Identifying therapeutic agents for amelioration of mitochondrial clearance disorder in neurons of familial Parkinson disease. Stem Cell Reports. 14:1060–1075. DOI:
10.1016/j.stemcr.2020.04.011. PMID:
32470327. PMCID:
PMC7355139.
138. Panagiotakopoulou V, Ivanyuk D, De Cicco S, et al. 2020; Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat Commun. 11:5163. DOI:
10.1038/s41467-020-18755-4. PMID:
33057020. PMCID:
PMC7560616.
139. Ke M, Chong CM, Zeng H, et al. 2020; Azoramide protects iPSC-derived dopaminergic neurons with PLA2G6 D331Y mutation through restoring ER function and CREB signaling. Cell Death Dis. 11:130. DOI:
10.1038/s41419-020-2312-8. PMID:
32071291. PMCID:
PMC7028918.
140. Yun W, Kim YJ, Lee G. 2022; Direct conversion to achieve glial cell fates: oligodendrocytes and Schwann cells. Int J Stem Cells. 15:14–25. DOI:
10.15283/ijsc22008. PMID:
35220289. PMCID:
PMC8889328.
141. Kwak TH, Kang JH, Hali S, et al. 2020; Generation of homogeneous midbrain organoids with
in vivo-like cellular composition facilitates neurotoxin-based Parkinson's disease modeling. Stem Cells. 38:727–740. DOI:
10.1002/stem.3163. PMID:
32083763.
142. Becerra-Calixto A, Mukherjee A, Ramirez S, et al. 2023; Lewy body-like pathology and loss of dopaminergic neurons in midbrain organoids derived from familial Parkinson's disease Patient. Cells. 12:625. DOI:
10.3390/cells12040625. PMID:
36831291. PMCID:
PMC9954141.
143. Ke M, Chong CM, Zhu Q, et al. 2021; Comprehensive perspectives on experimental models for Parkinson's disease. Aging Dis. 12:223–246. DOI:
10.14336/AD.2020.0331. PMID:
33532138. PMCID:
PMC7801282.
144. Pons-Espinal M, Blasco-Agell L, Consiglio A. 2021; Dissecting the non-neuronal cell contribution to Parkinson's disease pathogenesis using induced pluripotent stem cells. Cell Mol Life Sci. 78:2081–2094. DOI:
10.1007/s00018-020-03700-x. PMID:
33210214. PMCID:
PMC7966189.
145. Kim T, Song JJ, Puspita L, Valiulahi P, Shim JW, Lee SH. 2017;
In vitro generation of mature midbrain-type dopamine neurons by adjusting exogenous Nurr1 and Foxa2 expressions to their physiologic patterns. Exp Mol Med. 49:e300. DOI:
10.1038/emm.2016.163. PMID:
28280264. PMCID:
PMC5382556.
146. Nolbrant S, Heuer A, Parmar M, Kirkeby A. 2017; Generation of high-purity human ventral midbrain dopaminergic progenitors for
in vitro maturation and intracerebral transplantation. Nat Protoc. 12:1962–1979. DOI:
10.1038/nprot.2017.078. PMID:
28858290.
147. Han JJ. 2023; FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif Organs. 47:449–450. DOI:
10.1111/aor.14503. PMID:
36762462.
150. Liu GH, Ding Z, Izpisua Belmonte JC. 2012; iPSC technology to study human aging and aging-related disorders. Curr Opin Cell Biol. 24:765–774. DOI:
10.1016/j.ceb.2012.08.014. PMID:
22999273.
151. Miller JD, Ganat YM, Kishinevsky S, et al. 2013; Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell. 13:691–705. DOI:
10.1016/j.stem.2013.11.006. PMID:
24315443. PMCID:
PMC4153390.
152. Luk KC, Song C, O'Brien P, et al. 2009; Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 106:20051–20056. DOI:
10.1073/pnas.0908005106. PMID:
19892735. PMCID:
PMC2785290.
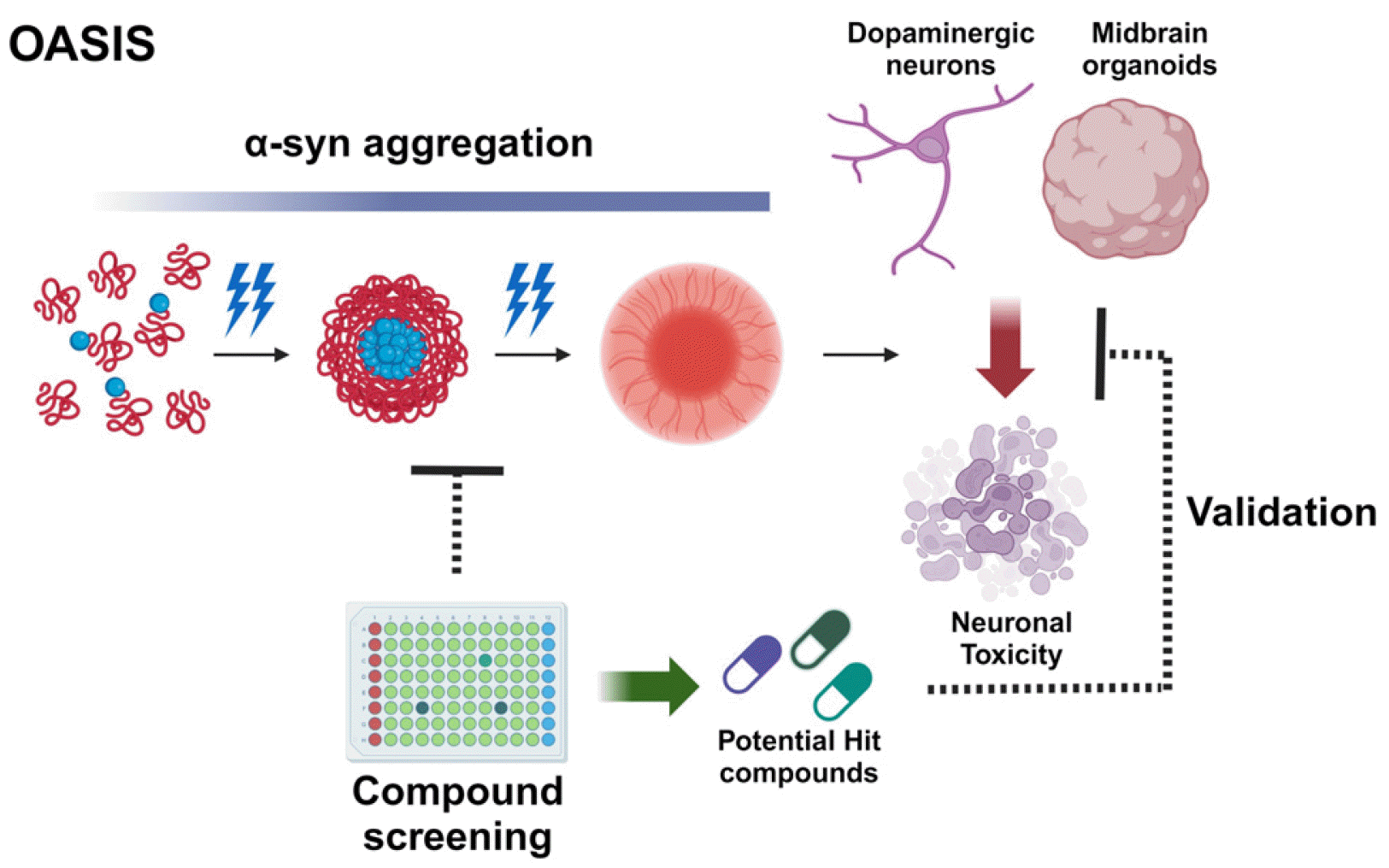
153. Kim MS, Ra EA, Kweon SH, et al. 2023; Advanced human iPSC-based preclinical model for Parkinson's disease with optogenetic alpha-synuclein aggregation. Cell Stem Cell. 30:973–986.e11. DOI:
10.1016/j.stem.2023.05.015. PMID:
37339636. PMCID:
PMC10829432.
154. Ra EA, Kim MS, Lee G. 2023; Optogenetic induction of alpha-synuclein aggregation in human dopaminergic neurons to model Parkinson's disease pathology. STAR Protoc. 4:102609. DOI:
10.1016/j.xpro.2023.102609. PMID:
37742181. PMCID:
PMC10522986.
156. Kim S, Kwon SH, Kam TI, et al. 2019; Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron. 103:627–641.e7. DOI:
10.1016/j.neuron.2019.05.035. PMID:
31255487. PMCID:
PMC6706297.
157. Ettle B, Kuhbandner K, Jörg S, Hoffmann A, Winkler J, Linker RA. 2016; α-Synuclein deficiency promotes neuroinflammation by increasing Th1 cell-mediated immune responses. J Neuroinflammation. 13:201. DOI:
10.1186/s12974-016-0694-4. PMID:
27565429. PMCID:
PMC5002168.
158. Desplats P, Lee HJ, Bae EJ, et al. 2009; Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 106:13010–13015. DOI:
10.1073/pnas.0903691106. PMID:
19651612. PMCID:
PMC2722313.
159. Magistrelli L, Contaldi E, Comi C. 2021; The impact of SNCA variations and its product alpha-synuclein on non-motor features of Parkinson's disease. Life (Basel). 11:804. DOI:
10.3390/life11080804. PMID:
34440548. PMCID:
PMC8401994.
160. Siddiqui IJ, Pervaiz N, Abbasi AA. 2016; The Parkinson disease gene SNCA: evolutionary and structural insights with pathological implication. Sci Rep. 6:24475. DOI:
10.1038/srep24475. PMID:
27080380. PMCID:
PMC4832246.
161. Hallacli E, Kayatekin C, Nazeen S, et al. 2022; The Parkinson's disease protein alpha-synuclein is a modulator of processing bodies and mRNA stability. Cell. 185:2035–2056.e33. DOI:
10.1016/j.cell.2022.05.008. PMID:
35688132. PMCID:
PMC9394447.
163. Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. 2016; α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A. 113:1931–1936. DOI:
10.1073/pnas.1520335113. PMID:
26839413. PMCID:
PMC4763774.
164. Fonseca-Ornelas L, Viennet T, Rovere M, et al. 2021; Altered conformation of α-synuclein drives dysfunction of synaptic vesicles in a synaptosomal model of Parkinson's disease. Cell Rep. 36:109333. DOI:
10.1016/j.celrep.2021.109333. PMID:
34233191. PMCID:
PMC8552450.
165. Kurapati S, Sadaoka T, Rajbhandari L, et al. 2017; Role of the JNK pathway in varicella-zoster virus lytic infection and reactivation. J Virol. 91:e00640–e00617. DOI:
10.1128/JVI.00640-17. PMID:
28637759. PMCID:
PMC5553188.
166. Che YH, Choi IY, Song CE, et al. 2023; Peripheral neuron-organoid interaction induces colonic epithelial differentiation via non-synaptic substance P secretion. Int J Stem Cells. 16:269–280. DOI:
10.15283/ijsc23026. PMID:
37385635. PMCID:
PMC10465334.
167. Susaimanickam PJ, Kiral FR, Park IH. 2022; Region specific brain organoids to study neurodevelopmental disorders. Int J Stem Cells. 15:26–40. DOI:
10.15283/ijsc22006. PMID:
35220290. PMCID:
PMC8889336.
168. Jang H, Kim SH, Koh Y, Yoon KJ. 2022; Engineering brain organoids: toward mature neural circuitry with an intact cytoar-chitecture. Int J Stem Cells. 15:41–59. DOI:
10.15283/ijsc22004. PMID:
35220291. PMCID:
PMC8889333.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download