This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Emergency pediatric living donor liver transplantation (LDLT) is vital for acute liver failure patients in life-threatening situations. However, we do not know the outcomes of emergency pediatric ABO-incompatible (ABOi)-LDLT, which is an alternative treatment for patients without ABO-compatible (ABOc) living liver donors. The purpose of our study is to compare the outcomes between emergency pediatric ABOi-LDLT and emergency pediatric ABOc-LDLT using data from the Korean Network for Organ Sharing (KONOS).
Methods
We analyzed retrospective KONOS data for consecutive pediatric emergency LDLT patients between 2017 and 2021 in Korea.
Results
The incidence of ABOc-LDLT and ABOi-LDLT was 83% (n=44) and 17% (n=9), respectively. The baseline, pre-transplant care, postoperative complications, and infectious complications of ABOi-LDLT did not differ from those of ABOc-LDLT. Graft survival and overall survival at 5 years in ABOi-LDLT were 100% and 100%, respectively, which was better than in ABOc-LDLT, but the survival difference between the two groups was not significant. No developed acute rejection in ABOi-LDLT patients. No factors in the multivariate analysis were related to patient mortality or graft failure. Neither graft failure nor death was associated with ABOi-LDLT.
Conclusion
This study concludes that emergency pediatric ABOi-LDLT is safe and feasible for use in ALF patients as well as other urgent instances.
Keywords: Living donors, Donor selection, Survival, Blood group incompatibility
INTRODUCTION
In patients without any previous history of liver disease, acute liver failure (ALF) is a potentially fatal consequence of acute liver injury that can cause coagulopathy, altered mental status, and frequent multiorgan dysfunction [
1]. It can impact almost every organ system and has a wide range of clinical manifestations and etiologies. Patients with chronic liver illness can have abrupt worsening in the form of acute-on-chronic liver failure (ACLF), which has a significant short-term death rate ranging from 14.6% to 78.6% [
2]. For children with ACLF or ALF who do not respond to medical treatment, liver transplantation (LT) is the last choice [
3]. Because the rapid worsening of liver function in patients with ALF or ACLF can be deadly, LT must be administered as soon as feasible [
4,
5].
For children with ALF or ACLF, emergency living donor liver transplantation (LDLT) could be considered even critically ill individuals have a high chance of receiving rapid DDLT in places with high rates of deceased organ donation. However, LDLT could be the only treatment option available to save infants who are dying of ALF or ACLF in places like Korea where there is a dearth of organ donors. In Korea, living liver donors (LLDs) are only allowed to be close relatives. Due to the high risk of antibody-mediated rejection (AMR), ABO-incompatible (ABOi)-LDLT was once thought to be contraindicated for pediatric patients. However, due to reports that its outcomes are comparable to those from ABO-compatible LDLT (ABOc-LDLT), its usefulness has recently been reopened [
6]. When an ABOc LLD is unavailable, an ABOi LLD ought to be taken into consideration.
Pre-transplant desensitization is typically required for most potential recipients of ABOi-LDLT who are older than one year in order to lower the risk of hyperacute rejection and AMR. This process typically takes two to three weeks [
7]. Time constraints, however, may make it impractical to implement those methods in emergency LDLT conditions for ALF or ACLF. Prior studies on emergency ABOi-LT have not demonstrated positive results or provided agreement on the best desensitization approach. Nevertheless, no prior research has conducted a comparison between emergency pediatric ABOc-LDLT and emergency pediatric ABOi-LDLT.
Therefore, the purpose of our study is to compare the outcomes between emergent pediatric ABOi-LDLT and emergent pediatric ABOc-LDLT.
MATERIALS AND METHODS
Study Design
Retrospective Korean Network for Organ Sharing (KONOS) data for consecutive pediatric emergency patients who received LDLT from 2017 to 2021 was investigated. The KONOS provided the patient list, demographics, and dates of death or LT, which were combined with the necessary lab data from every center. Fifty-three emergency pediatric LDLTs were conducted between 2017 and 2021. The Samsung Medical Center Institutional Review Board gave its approval to this study (SMC-2022-11-057-002). Informed consent was not required because this was a retrospective investigation into de-identified data.
Data Collection
Every patient’s sex, age, body mass index, pediatric end-stage liver disease scores, graft-to-recipient weight ratio, postoperative complications, graft failure, and mortality were recorded for every patient in KONOS and every institution. Pre-transplant ventilator support, pre-transplant intensive care unit (ICU) admission, hepatorenal syndrome, and grade of hepatic encephalopathy were the categorical variables that we evaluated in order to characterize the severity of the patient. ABOi between the recipient and donor blood types and re-transplantation were also evaluated. The time gap between the day KONOS received the emergency LDLT application and the day of transplantation was also assessed.
Administrative LDLT Approval Process in the Korea
KONOS approval is required prior to doing an LDLT. Documents pertaining to the donor and recipient status, such as an identification, consultation report, and statement of purpose, must be submitted by the transplantation center (
Supplementary Fig. 1). Following receipt and examination of those records, KONOS normally issues a decision either permission or disapproval within 14 days.
It can be difficult to explain a practical judgment on an emergency application of LDLT based only on laboratory data or medical records. Each patient must have a note from their physician and receive clearance from a government agency under Korea’s current LT application process.
Statistical Analysis
For continuous data, the patient features are shown as the median and range; for categorical variables, they are shown as the frequency and percentage. The chi-square test or Fisher’s exact test was used to compare categorical data, while the Mann–Whitney U-test was used to analyze continuous data. A Cox regression analysis was conducted in order to determine the risk factors for mortality and graft failure after emergency LDLT. Odds ratios and 95% confidence intervals were computed. All clinically meaningful potential risk variables were included in the multivariate analysis, and the final decision was made using backward variable selection to determine statistical significance. Two-sided p-values less than 0.05 indicated statistical significance in the results. SAS analytic software version 9.4 (SAS Institute, Cary, NC, USA) and R software version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses.
Ethics Statement
The study was approved by the Samsung Medical Center Institutional Review Board (SMC-2023-11-038) and complied with the Declaration of Helsinki. The Institutional Review Board waived the need for patient consent because of the retrospective nature of this observational study and its use of data from patient medical records.
RESULTS
Baseline Characteristics of the Recipients
The incidence of ABOc-LDLT and ABOi-LDLT was 83% (n=44) and 17% (n=9), respectively. The primary cause of both ABOc-LDLT and ABOi-LDLT was ALF. ICU care was required for over 70% of patients as they awaited LDLT. Furthermore, approximately 30% of patients in both groups underwent continuous renal replacement therapy (CRRT) due to renal failure, and approximately 40% of patients in both groups required mechanical breathing for support. In both groups, the average wait time for emergency LDLT was one day (range, 0–7 days). There was no discernible difference in any of the recipient characteristics between the two groups (
Table 1).
Table 1
Comparison of pediatric recipients of emergency ABOc or ABOi LDLT
|
Total (n=53) |
ABOc LDLT (n=44) |
ABOi LDLT (n=9) |
p-value |
|
Sex (male) |
16 (36.4) |
6 (66.7) |
0.140 |
|
Liver disease progression |
|
|
0.722 |
|
Acute liver failure |
32 (72.7) |
7 (77.8) |
|
|
Acute on chronic liver failure |
9 (20.5) |
2 (22.2) |
|
|
Critical cirrhosis |
3 (6.8) |
0 (0) |
|
|
Age (yr) |
1.0 (0.3–18) |
1 (0.2–12) |
0.765 |
|
BMI |
17.6 (14.2–26.0) |
17.9 (14.6–19.8) |
0.950 |
|
Re-transplantation |
6 (13.6) |
0 (0) |
0.574 |
|
Hepatic encephalopathy |
|
|
0.535 |
|
None |
24 (54.5) |
6 (66.7) |
|
|
Grade I or II |
13 (29.5) |
2 (22.2) |
|
|
Grade III or IV |
7 (15.9) |
1 (11.1) |
|
|
Hepatorenal syndrome |
4 (9.1) |
0 (0) |
0.347 |
|
Pre-transplant ICU care |
32 (72.7) |
6 (66.7) |
0.701 |
|
Pre-transplant ICU stay (day) |
3 (1–14) |
3 (1–5) |
0.656 |
|
Pre-transplant ventilator support |
18 (40.9) |
4 (44.4) |
0.845 |
|
Pre-transplant CRRT |
12 (27.3) |
3 (33.3) |
0.713 |
|
Ascites |
21 (47.7) |
1 (11.1) |
0.037 |
|
PELD score |
25 (10–40) |
33 (22–40) |
0.463 |
|
PELD score >30 |
19 (43.2) |
4 (44.4) |
0.989 |
|
Wait time (day) |
1 (0–7) |
1 (0–7) |
0.732 |
|
GRWR |
2.09 (0.86–4.99) |
1.75 (0.87–3.18) |
0.462 |

Characteristics of Living Liver Donors
Ninety-nine LLDs (92.5%) were the parents of the children in need of transplantation. In the ABOc-LDLT group, their median age ranged from 28 to 50 years, while in the ABOi-LDLT group, it was 35 years, with a range of 25 to 46 years. Compared to ABOc-LDLT, a larger percentage of robotic donor hepatectomy was performed in ABOi-LDLT. Following donor hepatectomy, the median hospital stays and follow-up periods for both groups were 8 days and about 1 year, respectively. There was no difference in any LLD characteristic between the two groups (
Table 2).
Table 2
Comparison of living liver donors for emergency pediatric ABOc and ABOi LDLT
|
Total (n=53) |
ABOc LDLT (n=44) |
ABOi LDLT (n=9) |
p-value |
|
Sex (male) |
15 (34.1) |
1 (11.1) |
0.248 |
|
Age (yr) |
37 (28–50) |
35 (25–46) |
0.765 |
|
BMI |
22.1 (17.5–29.4) |
22.8 (21.3–32.4) |
0.427 |
|
HTN |
1 (2.3) |
0 (0) |
0.648 |
|
Donor and recipient relationship |
|
|
0.572 |
|
Parents |
40 (90.9) |
9 (100) |
|
|
Relatives |
4 (9.1) |
0 (0) |
|
|
Donor operation |
|
|
0.018 |
|
Open |
21 (47.7) |
1 (11.1) |
|
|
Laparoscopic |
10 (22.7) |
1 (11.1) |
|
|
Robotic |
12 (27.3) |
7 (77.8) |
|
|
Postoperative complications |
4 (9.1) |
1 (11.1) |
0.780 |
|
Hospitalization |
8 (4–17) |
8 (4–13) |
0.958 |
|
Follow-up duration (day) |
365 (13–1,265) |
368 (43–382) |
0.999 |

Recipient Outcomes
Complications following LDLT were common in individuals. In the ABOc-LDLT group, the incidence of infectious complications was 52.3%, while in the ABOi-LDLT group, it was 66.7%. It’s interesting to note that the ABOi-LDLT group had the viral infection at a higher rate than the ABOc-LDLT group. The two groups’ median hospitalization periods were identical. During follow-up, two patients in the ABOc-LDLT group were dead.
For the ABOc-LDLT group, the median follow-up period was 26.9 months (range, 0–67.1 months), while for the ABOi-LDLT group, it was 22.3 months (range, 0.9–66.9 months). In the ABOc-LDLT group, diagnoses of acute cellular rejection (ACR) (n=6) and AMR (n=1) were made. During follow-up, graft failure emerged in seven patients and mortality occurred in eight patients, respectively. There was no discernible difference in recipient outcomes between the two groups (
Table 3). Although the ABOi-LDLT group outperformed the ABOc-LDLT group in terms of graft survival and overall survival, the difference was not statistically significant (
Fig. 1).
Figure 1
(A) Graft survival and (B) overall survival. ABOc, ABO-compatible; LDLT, living donor liver transplantation; ABOi, ABO-incompatible.
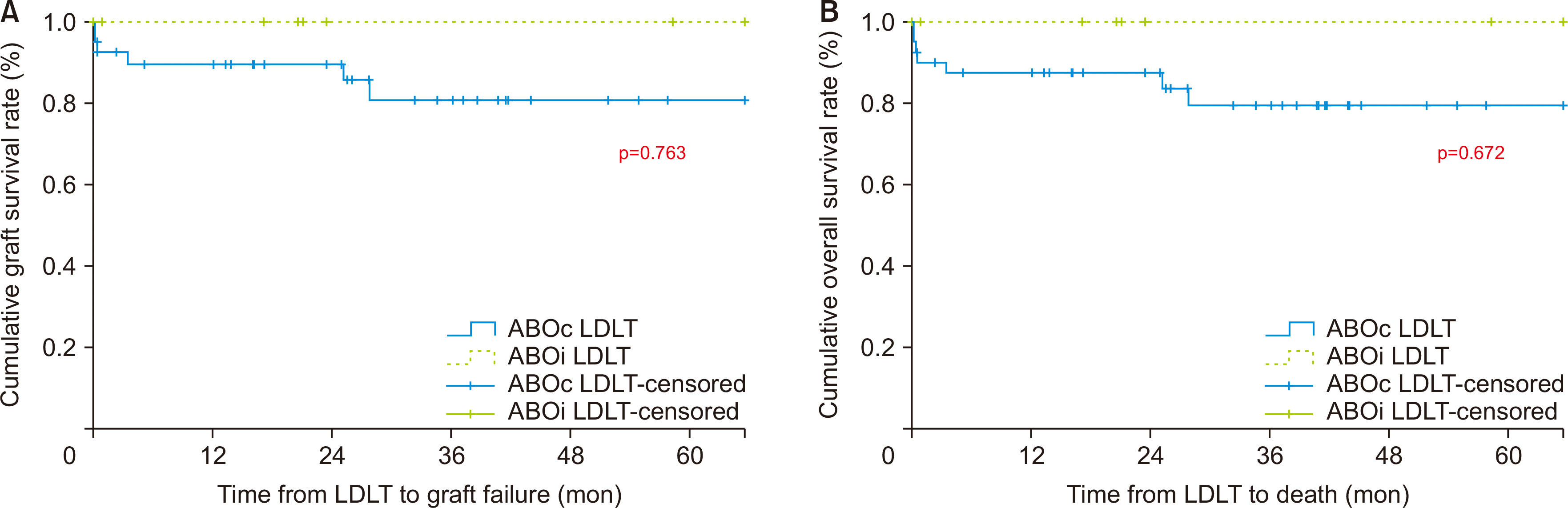

Table 3
Recipient outcomes from emergency pediatric ABOc and ABOi LDLT
|
Total (n=53) |
ABOc LDLT (n=44) |
ABOi LDLT (n=9) |
p-value |
|
Post-transplant ICU stay (day) |
8 (3–119) |
6 (2–18) |
0.159 |
|
Post-operative complications |
34 (77.3) |
8 (88.9) |
0.665 |
|
Post-transplant infectious complications |
23 (52.3) |
6 (66.7) |
0.487 |
|
Viral infection |
12 (27.3) |
5 (55.6) |
0.126 |
|
Bacterial infection |
15 (34.1) |
4 (44.4) |
0.706 |
|
Fungal infection |
7 (15.9) |
0 (0) |
0.334 |
|
Hospitalization (day) |
25 (1–78) |
24 (2–42) |
0.950 |
|
In hospital mortality |
2 (4.5) |
0 (0) |
0.514 |
|
Rejection |
|
|
0.438 |
|
None |
37 (84.1) |
9 (100) |
|
|
ACR |
6 (13.6) |
0 (0) |
|
|
AMR |
1 (2.3) |
0 (0) |
|
|
Graft failure |
6 (13.6) |
1 (11.1) |
0.838 |
|
Death |
7 (15.9) |
1 (11.1) |
0.714 |
|
Cause of death |
|
|
0.330 |
|
Postoperative complications |
1 (2.3) |
0 (0) |
|
|
Graft failure |
1 (2.3) |
0 (0) |
|
|
Infection |
4 (9.1) |
0 (0) |
|
|
Others |
1 (2.3) |
1 (11.1) |
|
|
Follow-up duration |
26.9 (0–67.1) |
22.3 (0.9–66.9) |
0.700 |

Risk Factors for Death and Graft Failure
The univariate analysis revealed a strong correlation between pre-transplant CRRT and hepatorenal syndrome and death. Moreover, in the univariate analysis, extended stays in the ICU following transplantation were linked to graft failure (
Table 4). In the multivariate analysis, no factors were found to be significant. Neither graft failure nor death was associated with ABOi-LDLT.
Table 4
Risk factors of pediatric patients receiving emergency LDLT
|
Univariate |
Patient mortality (n=53, events=8) |
|
Graft failure (n=53, events=7) |
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
|
Male sex |
1.38 (0.35–5.52) |
0.649 |
|
1.96 (0.44–8.77) |
0.378 |
|
Age |
1.03 (0.92–1.16) |
0.598 |
|
1.06 (0.94–1.19) |
0.355 |
|
BMI |
1.03 (0.80–1.31) |
0.841 |
|
1.06 (0.83–1.37) |
0.623 |
|
Ascites |
0.07 (0.00–1.44) |
0.085 |
|
0.20 (0.02–1.68) |
0.138 |
|
Reason for emergent LDLT |
|
|
|
|
|
|
Acute liver failure |
Reference |
1 |
|
Reference |
1 |
|
Acute-on-chronic liver failure |
0.75 (0.11–5.04) |
0.764 |
|
0.24 (0.01–5.61) |
0.373 |
|
Pre-transplant ICU care |
2.52 (0.31–20.45) |
0.389 |
|
2.05 (0.25–17.02) |
0.508 |
|
Pre-transplant ventilator care |
2.76 (0.66–11.55) |
0.166 |
|
1.24 (0.28–5.55) |
0.779 |
|
Pre-transplant CRRT |
4.90 (1.17–20.52) |
0.030 |
|
2.27 (0.51–10.16) |
0.283 |
|
Hepatorenal syndrome |
6.61 (1.32–33.03) |
0.021 |
|
1.33 (0.06–28.53) |
0.857 |
|
Hepatic encephalopathy |
|
|
|
|
|
|
Grade I or II |
2.13 (0.43–10.56) |
0.355 |
|
2.14 (0.43–10.60) |
0.352 |
|
Grade III or IV |
3.03 (0.51–18.18) |
0.226 |
|
1.46 (0.15–14.05) |
0.744 |
|
MELD score |
1.01 (0.94–1.09) |
0.763 |
|
1.07 (0.98–1.17) |
0.117 |
|
ABO-incompatibility |
1.09 (0.11–1.04) |
0.943 |
|
1.55 (0.14–17.11) |
0.719 |
|
Re-transplantation |
1.18 (0.14–9.57) |
0.879 |
|
0.53 (0.03–11.44) |
0.687 |
|
Post-transplant ICU stay |
1.02 (1.00–1.04) |
0.067 |
|
1.03 (1.00–1.05) |
0.041 |
|
GRWR |
0.38 (0.12–1.16) |
0.089 |
|
0.44 (0.16–1.23) |
0.118 |

DISCUSSION
LT is the only effective treatment for pediatric ALF patients; without it, they would not survive. Many centers have expanded their donor selection to include LDLT due to high waitlist mortality [
8,
9]. Early Western experience with high-urgency transplantation demonstrating inferior outcomes and ethical concerns about the emotional pressure that a high-urgency situation can impose on the LLD, potentially influencing their decision-making, are the main causes of the reluctance to do LDLT for ALF [
10].
Fifty-three pediatric patients underwent emergency LDLT in Korea during the research period, according to KONOS data. All ABOi-LDLT patients (n=9) lived for five years without developing AMR and ACR. Due to the limitation of available DDLT choices, LDLT is frequently utilized for the therapy of ALF in Korea. Excellent recipient survival and reported LLD safety have been documented [
11,
12]. In an emergency, ABOi LLDs will be the only option if ABOc LLDs are unavailable. By decreasing anti-blood type isoagglutinin and depleting B-cells, ABOi-LDLT can currently be carried out effectively and produce results comparable to ABOc-LDLT [
13,
14].
For patients with ALF or those admitted to the ICU, several centers expressly refrain from using ABOi-LDLT [
15]. Several factors make planning an ABOi-LDLT to treat ALF more difficult. There is not much time between deciding to transplant and doing the LT surgery. In both ABOc- and ABOi-LDLTs in our study, the median duration between the decision to transplant and the emergency LDLT was one day. In order to sufficiently reduce the B lymphocyte cohort for ABOi-LDLT desensitization, preoperative rituximab must be administered two to three weeks before the transplant. But waiting that long for LT is risky for an ALF patient because of rapid deterioration. Overcoming the ABO barrier in the limited time permitted for ABOi-LDLT for ALF is difficult. Because patients with multiple organ failure have a narrow period of urgency for transplantation, increasing donor availability and decreasing wait times are essential to improve patient outcomes.
Extracorporeal therapy and organ support are necessary for children with ALF, which raises their risk of infection complications. Thus, it is especially difficult to balance desensitization and infection in these patients. There is no information regarding desensitization therapy in our data. On the other hand, we presume that very little desensitization treatment was used given that the median wait duration was one day.
Emergency ABOi-LDLT is typically reserved for extreme cases and is not a routine operation. Early research on emergency ABOi-LT in the LDLT circumstances revealed a significant rate of complications from infections, graft loss by immunologic injury, and other vascular issues [
16]. Mendes et al. [
16] reported that five out of ten patients who underwent emergency ABOi-LT were still living at the time of publication and four of them had received original grafts. Nine months following the initial transplant, one patient had a second transplant. Infections (n=3), AMR (n=3), ACR (n=1), and biliary problems were the main consequences. Approximately 60% of emergency ABOi-LDLT patients had a bacterial or viral illness after transplantation. The median length of hospital stay was 24 days in our study; thus any additional issues were easily managed despite the infectious complications. Even though AMR-related graft loss is a significant factor in ABOi-LT, our investigation did not produce any AMR or ACR.
There are various restrictions on our investigation. First, selection bias was introduced when we used KONOS data to retrospectively assess emergency LDLT cases. We lacked extensive details regarding isoagglutinin titers and desensitization procedures. Second, there were only nine emergency pediatric ABO-LDLTs, which reduced our statistical power. Third, the circumstances around the transplantation center and the donor’s or family’s willingness can have an impact on whether or not emergency LDLT is received. These factors are hard to measure. Fourth, the etiology was not carefully assessed or examined within each subgroup, particularly with regard to whether the patient had ALF or ACLF.
This study concludes that emergency pediatric ABOi-LDLT is safe and feasible for use in ALF patients as well as other urgent instances.
ACKNOWLEDGEMENTS
The Korean National Health Insurance (NHIS) helped with data collection for this study, but the Korean NHIS and Korean NRF did not influence study design, data analysis, data interpretation, or drafting of the manuscript.
REFERENCES
1. European Association for the Study of the Liver. 2017; EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 66:1047–1081. DOI:
10.1016/j.jhep.2016.12.003. PMID:
28417882.
2. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. 2013; Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 144:1426–1437.e14379. DOI:
10.1053/j.gastro.2013.02.042. PMID:
23474284.

3. Lee S, Lee SK. 2019; Pediatric liver transplantation in Korea: long-term outcomes and allocations. J Korean Soc Transplant. 33:1–5. DOI:
10.4285/jkstn.2019.33.1.1.

4. Shingina A, Mukhtar N, Wakim-Fleming J, Alqahtani S, Wong RJ, Limketkai BN, et al. 2023; Acute liver failure guidelines. Am J Gastroenterol. 118:1128–1153. DOI:
10.14309/ajg.0000000000002340. PMID:
37377263.

6. Honda M, Sugawara Y, Kadohisa M, Shimata K, Sakisaka M, Yoshii D, et al. 2018; Long-term outcomes of ABO-incompatible pediatric living donor liver transplantation. Transplantation. 102:1702–1709. DOI:
10.1097/TP.0000000000002197. PMID:
29620615. PMCID:
PMC6166697.

7. Oh J, Kim JM. 2020; Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation. Clin Mol Hepatol. 26:1–6. DOI:
10.3350/cmh.2019.0023. PMID:
30909688. PMCID:
PMC6940481.

8. Moon DB, Lee SG, Kang WH, Song GW, Jung DH, Park GC, et al. 2017; Adult living donor liver transplantation for acute-on-chronic liver failure in high-model for end-stage liver disease score patients. Am J Transplant. 17:1833–1842. DOI:
10.1111/ajt.14198. PMID:
28097804. PMCID:
PMC5516156.

9. Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Sanefuji K, Kayashima H, et al. Living donor liver transplantation for acute liver failure: a 10-year experience in a single center. J Am Coll Surg. 2008; 206:412–418. DOI:
10.1016/j.jamcollsurg.2007.08.018. PMID:
18308209.

10. Campsen J, Blei AT, Emond JC, Everhart JE, Freise CE, Lok AS, et al. 2008; Outcomes of living donor liver transplantation for acute liver failure: the adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 14:1273–1280. DOI:
10.1002/lt.21500. PMID:
18756453. PMCID:
PMC3732478.

11. Ogura Y, Kabacam G, Singhal A, Moon DB. The role of living donor liver transplantation for acute liver failure. Int J Surg. 2020; 82S:145–148. DOI:
10.1016/j.ijsu.2020.04.058. PMID:
32353557.

12. Gupta A, Asrani SK. 2019; Role of living donor liver transplantation in acute liver failure. Liver Transpl. 25:1308–1309. DOI:
10.1002/lt.25610. PMID:
31344765.

13. Kim JM, Kwon CH, Joh JW, Han SB, Sinn DH, Choi GS, et al. 2016; Case-matched comparison of ABO-incompatible and ABO-compatible living donor liver transplantation. Br J Surg. 103:276–283. DOI:
10.1002/bjs.10048. PMID:
26695115.

14. Kim JM, Kwon CH, Joh JW, Kang ES, Park JB, Lee JH, et al. 2013; ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol. 59:1215–1222. DOI:
10.1016/j.jhep.2013.07.035. PMID:
23928408.

15. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. 2016; ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 16:157–170. DOI:
10.1111/ajt.13444. PMID:
26372830.

16. Mendes M, Ferreira AC, Ferreira A, Remédio F, Aires I, Cordeiro A, et al. 2013; ABO-incompatible liver transplantation in acute liver failure: a single Portuguese center study. Transplant Proc. 45:1110–1115. DOI:
10.1016/j.transproceed.2013.02.012. PMID:
23622639.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download