INTRODUCTION
CASE PRESENTATION
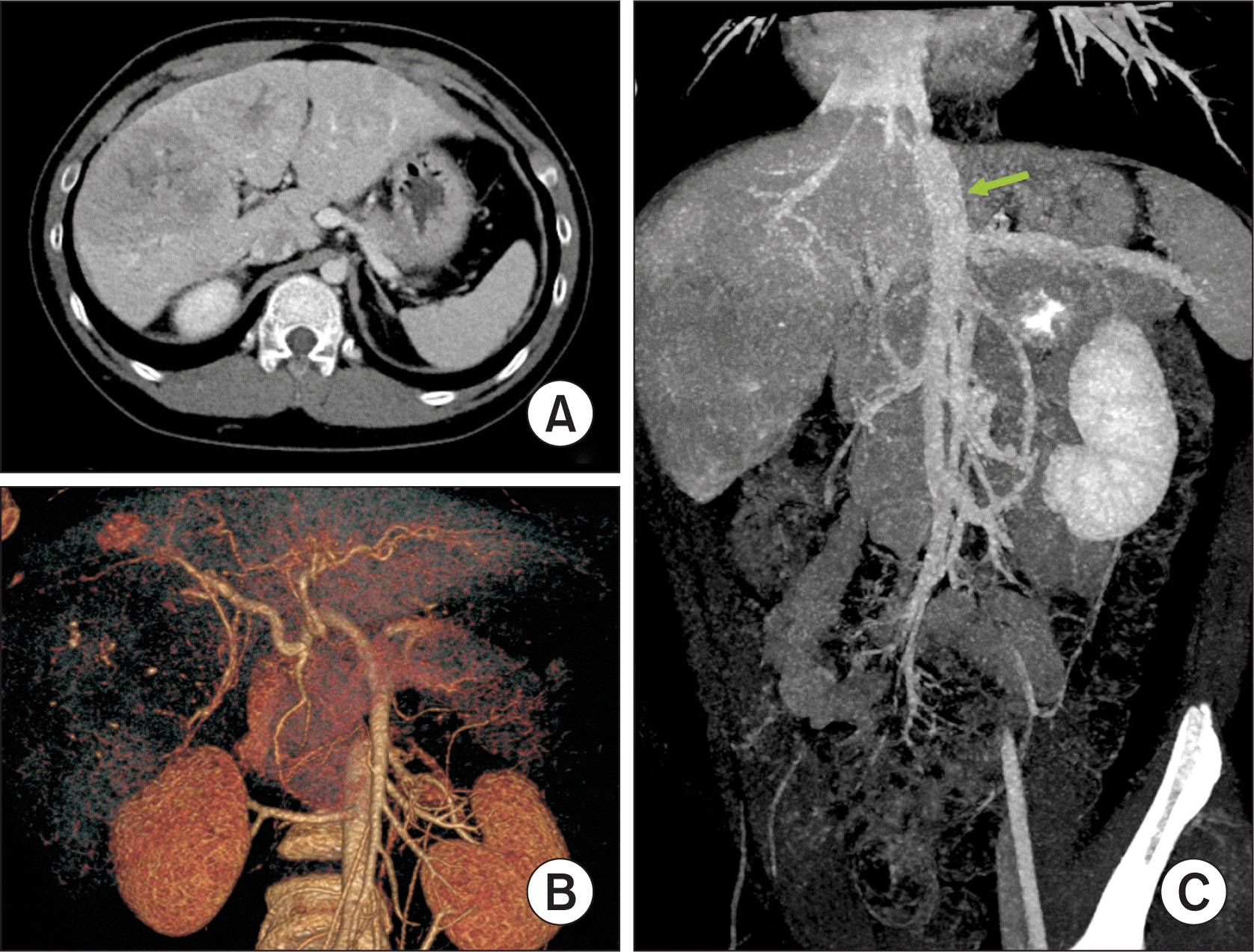 | Figure 1Preoperative and postoperative computed tomography findings of the donor. (A) A fissural vein was identified at the medial section (arrow). (B) A large segment VIII vein was identified (arrow). (C) Parenchymal transection plane was designed to preserved the segment VIII vein (dotted line). (D) Postoperative computed tomography taken at 7 days after surgery showed no significant hepatic venous congestion at the remnant right liver. |
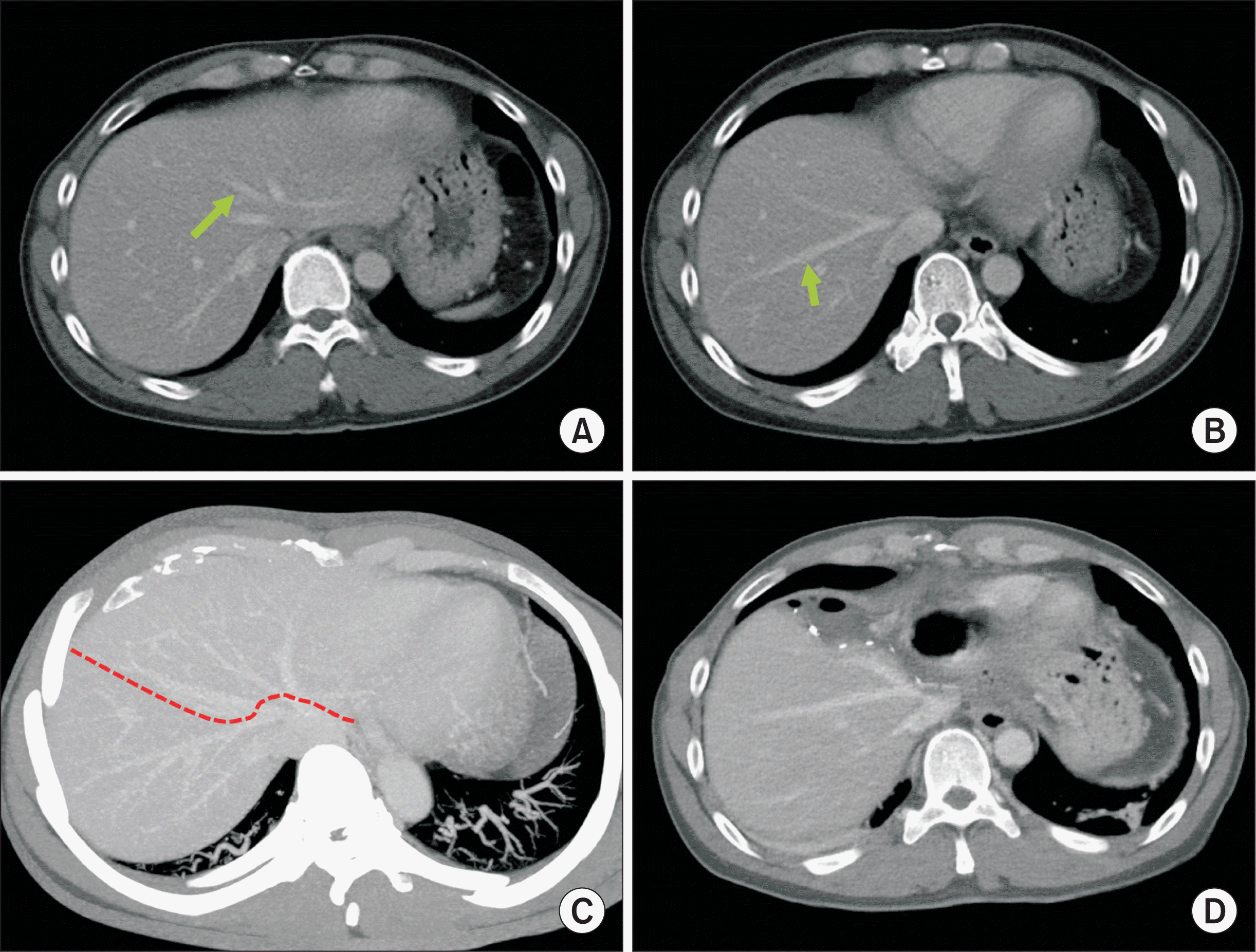 | Figure 2Pretransplant computed tomography findings of the recipient. (A) Multiple liver nodules were identified. (B) Hepatic arteries were enlarged without anatomical variation. (C) Congenital absence of the portal vein was identified with development of a large portocaval shunt in to the suprahepatic inferior vena cava (arrow). |
 | Figure 4Intraoperative photographs of bench work for a left liver graft. (A) A small-sized left hepatic vein orifice was identified (arrow). (B) Transected middle hepatic vein trunk was exposed at the liver cut surface (arrow). (C–D) An iliac vein segment was anastomosed with the middle hepatic vein trunk. (E) The inferior vena cava side of the conduit was anastomosed with the left hepatic vein stump. (F–H) An incision was made at the left lateral wall of the graft left hepatic vein, and patch venoplasty was performed to make a wide single outflow vein orifice. |
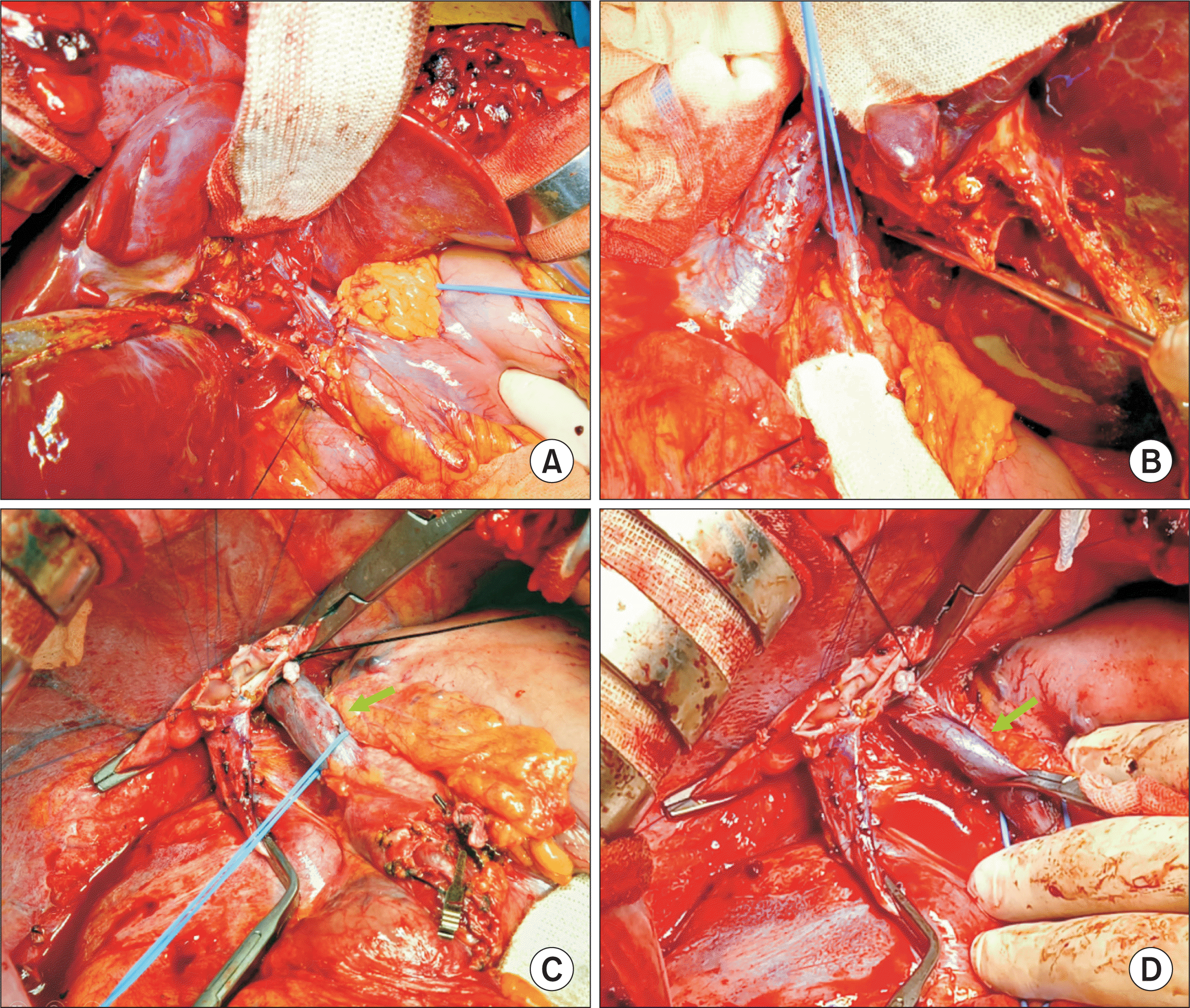
 | Figure 6Intraoperative photographs of the hepatic vein and portal vein reconstruction. (A) The graft hepatic vein was enlarged through unification venoplasty. (B) The isolated portocaval shunt vein was transected. (C–E) The liver graft was placed into the right subphrenic fossa, thus inducing dextrorotation and hepatic vein reconstruction was performed at that position. (F–H) The interposed iliac vein conduit at the graft portal vein was obliquely resected to match the length and axis of the excised portocaval shunt vein. A 1-cm-long wedge-shaped iliac vein segment (arrows) was inserted between the graft portal vein and the portocaval shunt vein stumps to prevent redundancy. |
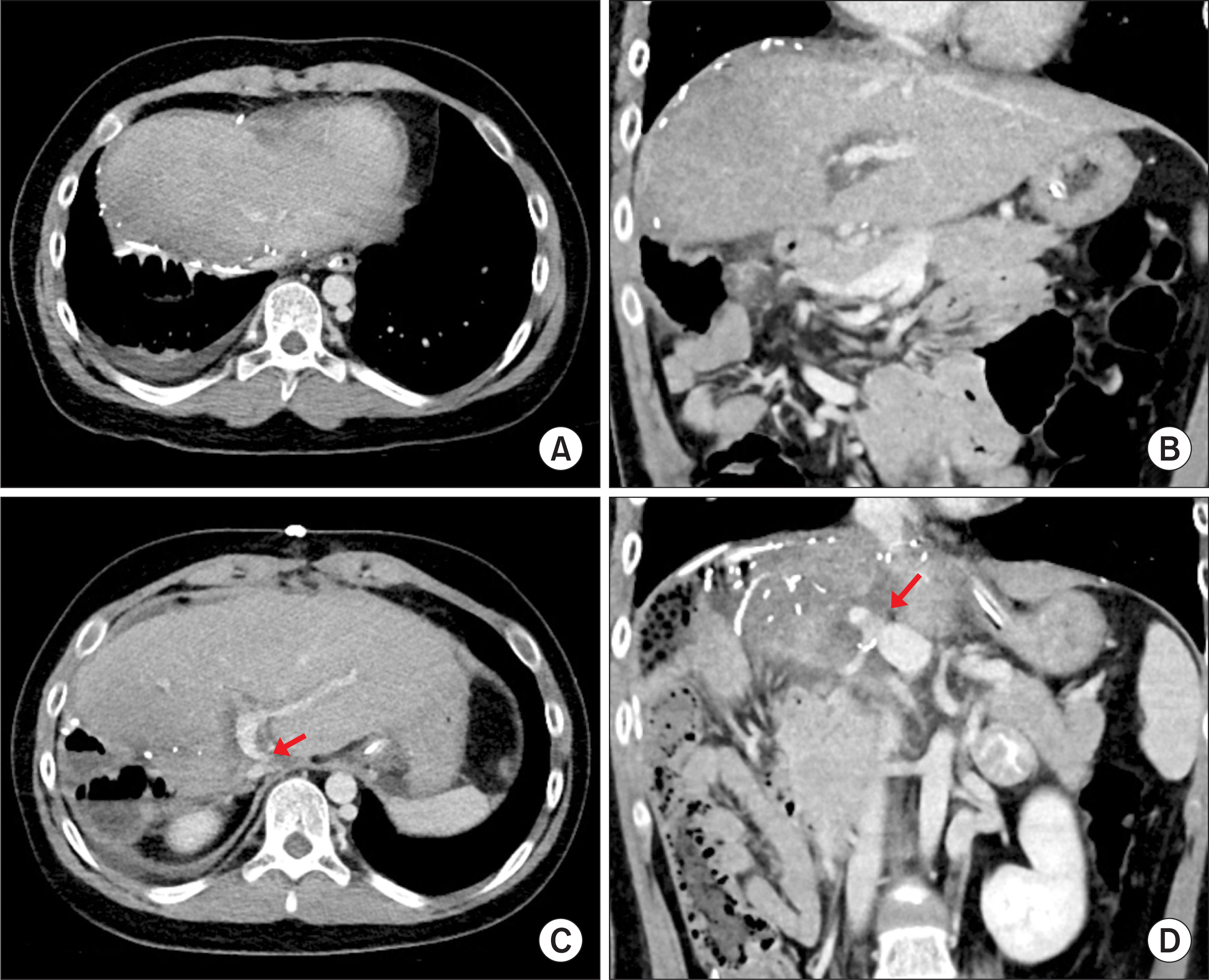 | Figure 8Posttransplant computed tomography scan taken at 4 days after transplantation. (A, B) Uneventful reconstruction of the graft hepatic vein was identified. (C, D) Portal vein anastomosis showed a slight anastomotic stenosis (arrows), with unusual location of the inflow portocaval shunt vein. |
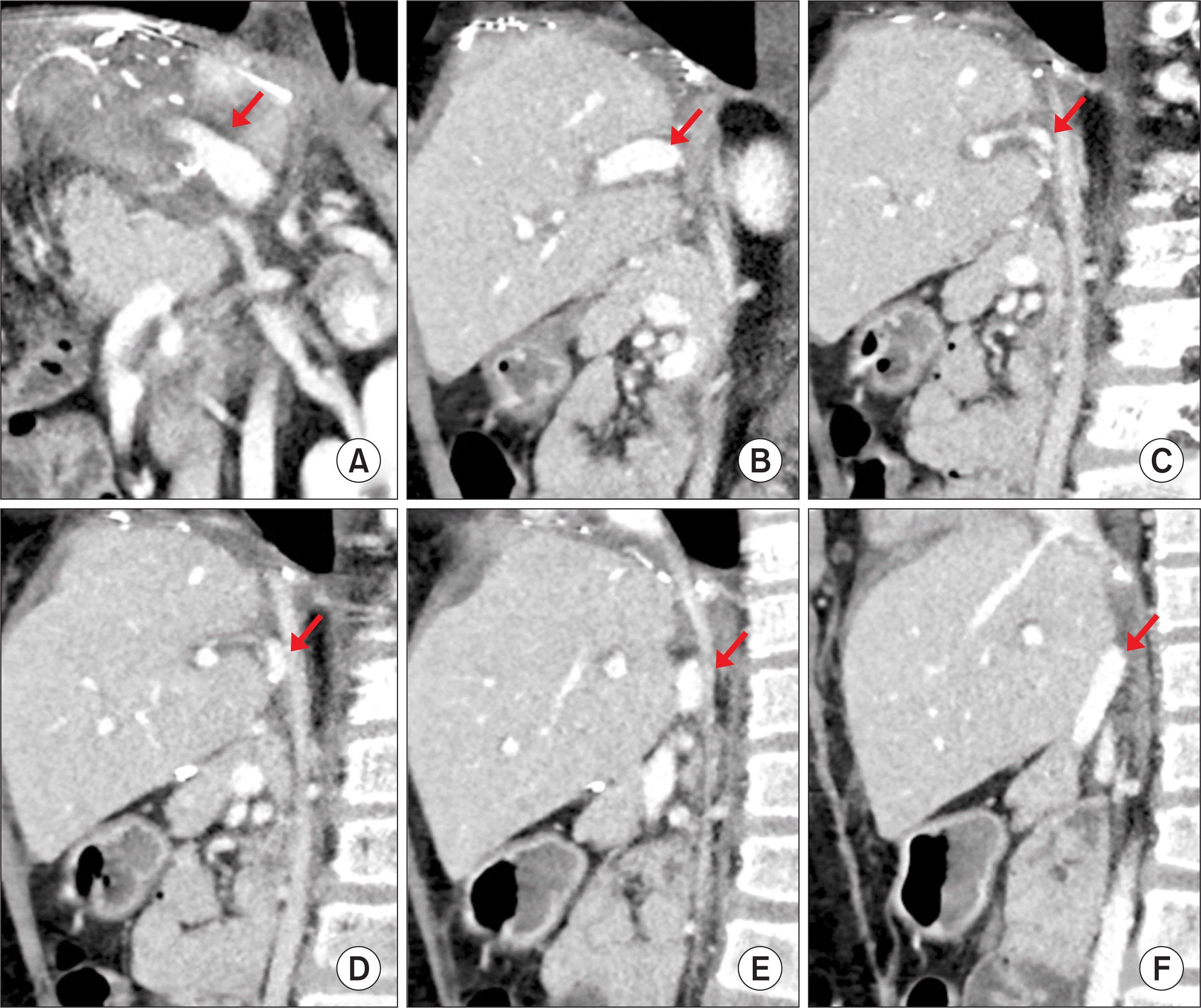 | Figure 9Posttransplant computed tomography scan taken at 14 days after transplantation. (A) Three-dimensional reconstruction of the portal vein shows stream-lined configuration (arrow). (B–F) The running course of the reconstructed portal vein shows the unusual location behind the liver graft (arrows). |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download