Abstract
Thyroid disorders are considered to be linked to various health issues, including gynecologic cancers. Studying this association is crucial in clinical practice. This approach was applied through searches in Scopus, WOS, PubMed, and Google Scholar. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist was followed. The quality assessment was checked. The meta-analyses were performed using R-4.3.2 (R Core Team, Vienna, Austria) and SPSS version 28 (SPSS Inc., Armonk, NY, USA). The results demonstrated that 19 studies investigated the association between thyroid disorders and gynecologic cancers in adult females. The studies were categorized into two groups: group 1 examined thyroid status in various gynecologic cancers, while group 2 comprised case-control studies examining gynecologic cancer incidence in females with thyroid disorders compared to control. Among females with gynecologic cancers, 13% (95% confidence interval [CI], 10–17%) had hypothyroidism. When comparing hypothyroidism and hyperthyroidism across studies, the overall percentage for hypothyroidism was 14% (95% CI, 9–22%), while for hyperthyroidism, it was 3% (95% CI, 2–5%). The odds ratio for hypothyroidism in females with uterine cancer was 2.65 (P<0.05). Additionally, hypothyroidism showed a significant risk ratio of 1.3 (P<0.05) for different gynecologic cancers. However, hyperthyroidism was significantly associated with increased ovarian cancer mortality (risk ratio [RR], 2.14; P=0.03); conversely, hypothyroidism showed no significant relationship (RR, 1.35; P=0.26). The findings concluded that hypothyroidism is significantly associated with various gynecologic cancers, suggesting a potential role in its pathogenesis. Conversely, hyperthyroidism is linked to an increased risk of ovarian cancer mortality. Further research is needed to clarify whether hyperthyroidism predisposes females to ovarian cancer.
Thyroid disorders, such as hypothyroidism and hyperthyroidism, are linked to various health issues because of their impact on metabolic rate, energy balance, and various cellular processes [1]. Disturbances in thyroid hormone secretion have long been recognized in many types of cancer [2]. Recently, the link between thyroid disorders and gynecologic cancers has been investigated to determine whether an association exists [3].
Gynecological cancers include malignancies affecting the ovaries, uterus, cervix, vagina, and vulva, collectively forming a diverse group of cancers that affect the female reproductive system. Cervical cancer is one of the most common gynecological cancers worldwide, whereas ovarian cancer, although considered less common, is more fatal than cervical and uterine cancers [4]. Uterine (endometrial) cancer is the predominant type of gynecological cancer in developed countries. Vaginal and vulvar cancers are relatively rare compared to cervical, ovarian, and uterine cancers [5].
While factors such as genetic predisposition, hormonal influences, metabolic syndrome [6], and environmental exposure have been studied, screening for thyroid disorders in females with these cancers and the potential role of thyroid disorders in predisposing females to these cancers have gained increasing attention [7].
Several studies have explored the association between thyroid disorders and gynecological cancers. Various studies have implicated hypothyroidism as a potential risk factor for gynecological malignancies [8]. Conversely, hyperthyroidism has shown varied or contrasting associations depending on the specific cancer type [9].
Other meta-analyses have investigated the relationship between breast cancer and thyroid levels [10] and between polycystic ovary syndrome and thyroiditis [11]. However, no study, except for ours, has focused exclusively on gynecologic cancers.
In the present review, the included studies were categorized into two groups: group 1 studies focused on the thyroid status of females diagnosed with various gynecologic cancers, whereas group 2 studies investigated the incidence of gynecological cancers in females with thyroid disorders compared to those without such disorders. Hypothyroidism appears to be potentially associated with uterine cancer, as supported by several studies. However, the role of hyperthyroidism in the incidence of ovarian cancer remains uncertain and warrants further clarification through additional research.
While initial findings suggest potential associations between thyroid disorders and gynecological cancers, further research is essential to elucidate these relationships, particularly concerning rare gynecological and ovarian cancers.
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.
A comprehensive search was conducted on May 5, 2024, across Scopus, Web of Science, PubMed, and Google Scholar using the search terms “thyroid level”, “hypothyroidism”, “hyperthyroidism”, and “gynecologic cancers”.
The studies included in this review met the following criteria. 1) They focused on gynecological cancers or tumors. 2) Investigated any type of thyroid disorder (hypothyroidism, hyperthyroidism, or both) in general. 3) Conducted on adult females. 4) Females with gynecological cancers or tumors screened for their thyroid status were included. And 5) females with thyroid disorders were included, and their risk of developing gynecological cancers was assessed. The exclusion criteria were as follows. 1) Studies that were not in English. 2) Studies with restricted full text. 3) Focus on breast cancer. And 4) patients with polycystic ovary syndrome or hyperplasia, which were not considered as tumors, were excluded.
Eligibility of the search results was assessed in two stages: title and abstract screening and followed by full-text screening.
The data extracted included author name, year of publication, country of study, journal of publication, mean age (years), study design, type of gynecological cancer or tumor, thyroid status, monocenter or multicenter status, total number of females with gynecological cancers (and controls in case of control studies), number of females with thyroid disorders (or vice versa per study design), total number of females with thyroid disorders and controls, and number of females who developed gynecological cancers. Quality assessment was performed using the Newcastle-Ottawa quality assessment scale (Supplementary Tables 1, 2) [12]. A rating of seven stars or higher was considered high quality, whereas ratings below seven stars were considered poor quality.
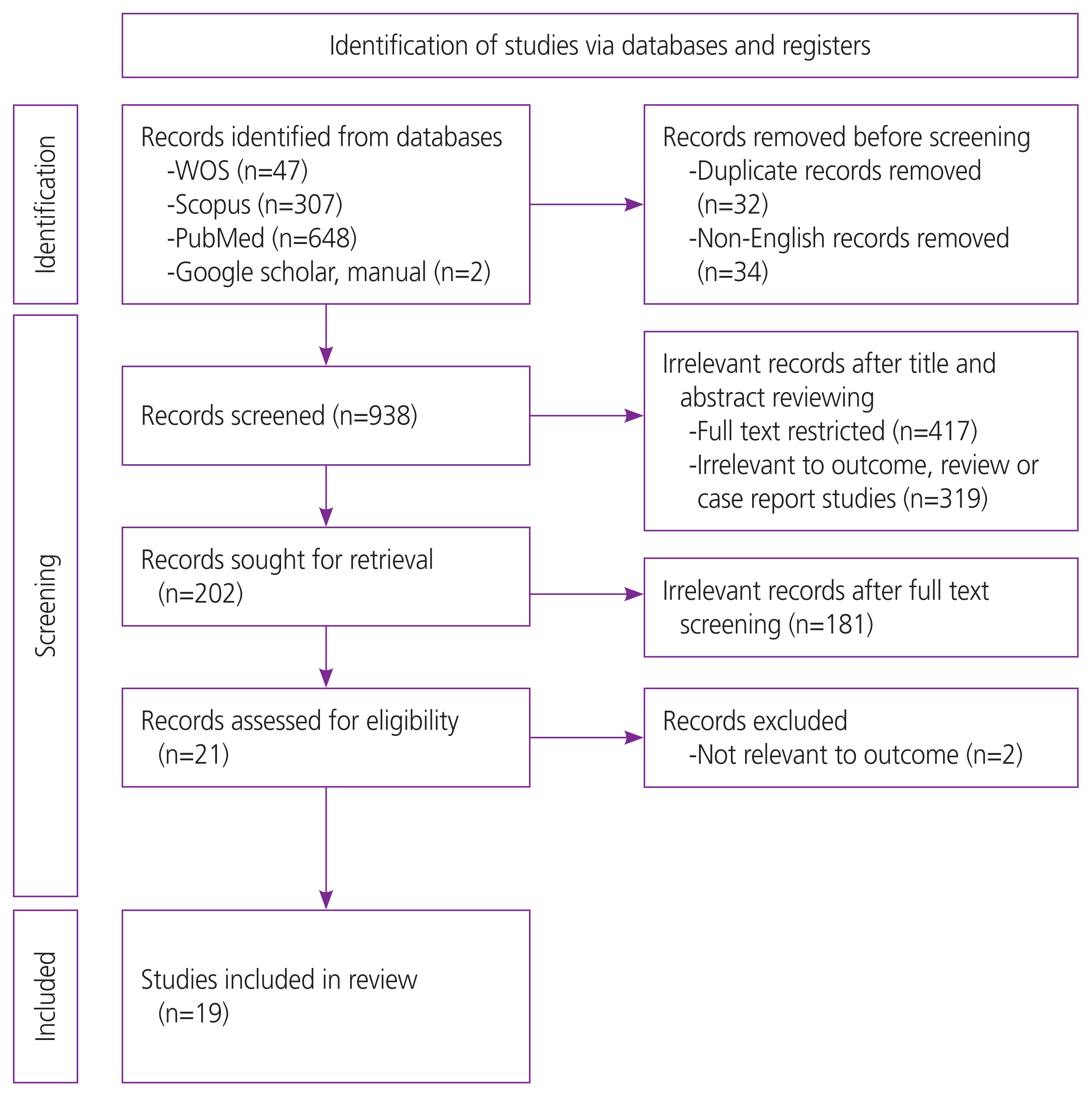
The initial search identified 47 studies from Web of Science, 307 from Scopus, and 648 from PubMed. Thirty-two studies were excluded because they were duplicates, and 34 studies were not published in English. A total of 938 studies were screened at the title and abstract stages. Of these, the full texts of 417 studies were unavailable, and 319 studies were deemed irrelevant. After full-text screening, 181 studies were excluded because they were irrelevant, and 21 studies were assessed for eligibility. The final analysis was performed on 19 included studies (Fig. 1).
Nineteen studies [3,7–9,13–27] were included in the final analysis. These studies were published between 2000 and 2024. The largest number of included studies was found in 2013 (four publications). The largest number of studies (five studies) were from authors in the USA. The Gynecologic Oncology journal published most of the studies (three studies) included in this study, followed by the British Journal of Cancer (two studies). All studies focused on adult females aged 20 years or older (Supplementary Table 3). The studies were categorized into two groups: group 1 consisted of 13 studies (2, 3, 5, 6, 7, 8, 9, 11, 12, 13, 14, 18, and 19) examining the thyroid status in females with various gynecological cancers (Table 1). Seven studies (5, 9, 11, 12, 13, 18, and 19) compared thyroid status between females with gynecological cancer and controls (Supplementary Table 4). Studies 5 and 18 broadly addressed thyroid disorders, whereas studies 3, 6, 9, 11, 12, 13, and 14 focused specifically on hypothyroidism. Studies 2, 7, 8, and 19 examined hypothyroidism and hyperthyroidism separately. No study focused solely on hyperthyroidism. Studies 2, 3, and 19 focused on ovarian cancer. Studies 5, 6, 7, 9, 11, 12, 13, and 14 focused on uterine cancer and tumors. Study eight focused on general gynecological cancers, whereas study 18 focused on ovarian and uterine cancers.
Group 1 studies that screened the thyroid status of females with various types of gynecological cancers
Group 2 (Supplementary Table 5) comprised six case-control studies (studies 1, 4, 10, 15, 16, and 17) that investigated the incidence of gynecological cancers in patients with thyroid disorders compared to control groups without thyroid disorders (Table 2). Study four focused on the incidence of cancer in females with hypothyroidism. Studies 1 and 17 examined cancer occurrence in females with hypothyroidism and hyperthyroidism, respectively, whereas study 16 focused on females with Graves’ disease, characterized by hyperthyroidism. Study 15 specifically targeted females with Hashimoto’s disease, which is associated with hypothyroidism. Study 10 particularly analyzed female cancer-related mortality rates. Study 1 involved screening for general gynecological cancers. Study 4 focused on cervical cancer, and study 10 involved screening for general gynecological and ovarian cancers. Studies 15 and 16 involved screening for uterine, cervical, and ovarian cancers, whereas study 17 focused on ovarian and uterine cancers.
Group 2 case-control studies that screened various types of gynecological cancers in females with thyroid disorders
Overall, studies 2, 3, 5, 6, 7, 8, 9, 11, 12, 13, 14, 18, and 19 included 33,337 females with various types of gynecological cancers, of whom 4,228 had thyroid disorders. The proportion of females with gynecological cancers and thyroid disorders was 8% (95% confidence interval [CI], 6–12%), as determined by a proportional meta-analysis (Supplementary Figs. 1, 2). Studies 2a, 3, 6, 7a, 8a, 9, 11, 12, 13, 14, and 19b included 26,952 females with various types of gynecological cancer, 4,031 of whom had hypothyroidism. The proportion of females with gynecological cancers and hypothyroidism was 13% (95% CI, 10–17%), as determined by proportional meta-analysis (Supplementary Figs. 3, 4). The proportion increased from 8% (95% CI, 6–12%) to 13% (95% CI, 10–17%), suggesting a greater association between hypothyroidism and gynecological cancers than between hyperthyroidism and general thyroid disorders. For uterine cancers and tumors, the proportion of females with hypothyroidism was 13% (95% CI, 9–18%) (Supplementary Figs. 5, 6). When comparing the proportions of hypothyroidism and hyperthyroidism in studies that screened for both conditions (Table 3), the overall percentage of hypothyroidism was 14% (95% CI, 9–22%), whereas the overall percentage of hyperthyroidism was 3% (95% CI, 2–5%) (Supplementary Figs. 7, 8). This further indicates a strong association between hypothyroidism and gynecological cancers than between hyperthyroidism and gynecological cancers.
Studies screening both hypothyroidism and hyperthyroidism in females with various types of gynecological cancers
For case-control studies that investigated the thyroid status in females with various types of gynecological cancers (Table 4), the overall odds ratio (OR) was determined (Supplementary Figs. 9, 10), revealing that for hypothyroidism, hyperthyroidism, and thyroid disorders in general, the overall OR was 1.56 with a P-value of 0.03. This indicates that females with gynecological cancers have 1.56-fold greater odds of developing thyroid disorders than those without gynecological cancers.
Case-control studies that compared the thyroid status of females with different types of gynecological cancers versus controls
While examining hypothyroidism, specifically in females with uterine cancer or tumors, the overall OR was determined (Supplementary Figs. 11, 12). The overall OR was 2.65 with P-value <0.05, and I2 decreased from 93% to 66%, indicating reduced heterogeneity. This suggests that women with uterine cancer have 2.56 times greater odds of developing hypothyroidism than those without uterine cancer.
For case-control studies (studies 1, 4, 15, 16, and 17) that assessed the incidence of any type of gynecological cancer in patients with thyroid disorders compared to control groups without thyroid disorders, the overall risk ratio was determined (Supplementary Figs. 13, 14). The results showed that for all hypothyroidism, hyperthyroidism, and thyroid disorders in general, the overall risk ratio was 1.12, with a P-value of 0.27, indicating a slight, non-significant increase in the risk of gynecological cancers among females with any thyroid disorder.
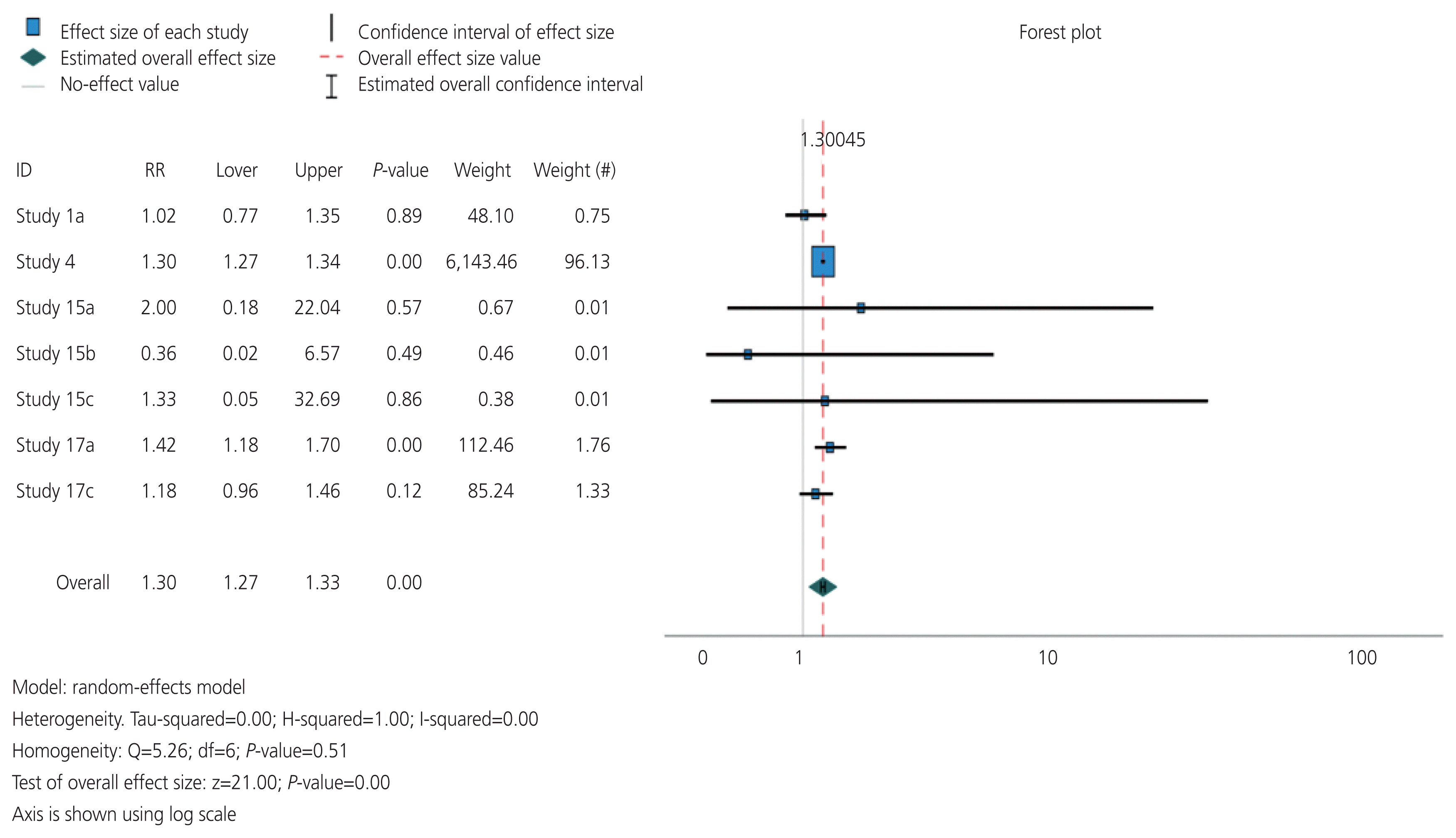
The overall risk ratio was determined by specifically examining the incidence of different gynecological cancers in females with hypothyroidism compared with those in the control group without hypothyroidism (Fig. 2). The overall risk ratio was 1.3, with a P-value <0.05, indicating that females with hypothyroidism have a 1.3-fold greater risk of developing gynecological cancers than those without hypothyroidism. Additionally, I2 decreased from 87% to 0%, indicating no heterogeneity across studies. This suggests that hypothyroidism poses a significantly greater risk than hyperthyroidism or other thyroid disorders.
In contrast, when examining the incidence of different gynecological cancers in females with hyperthyroidism compared to those in the control group without hyperthyroidism (Supplementary Figs. 15, 16), the overall risk ratio was 0.87, with a P-value of 0.62, indicating a non-significant decrease in the risk of gynecological cancers among females with hyperthyroidism.
Study 10 was a case-control study that examined the incidence of ovarian cancer mortality and mortality because of female genital tract cancer, excluding ovarian cancer, in females with hypothyroidism and hyperthyroidism compared to the control group without thyroid disorders (Table 5). The risk ratio for ovarian cancer mortality in females with hyperthyroidism was 2.14 (P=0.03), indicating a 2.14-fold greater risk in females with hyperthyroidism than in those without hyperthyroidism. However, the risk ratio for ovarian cancer mortality in females with hypothyroidism was not significantly greater than that in females without hypothyroidism (risk ratio [RR], 1.35; P=0.26). The risk ratios for mortality because of female genital tract cancers, excluding ovarian cancers, in females with hyperthyroidism and hypothyroidism were 1.08 (P=0.91) and 1.09 (P=0.83), respectively, suggesting a non-significant increase in the risk of mortality because of these types of cancer.
Study 10 screening cancer mortality in females with thyroid disorders
This systematic meta-analysis synthesized findings from 19 studies to investigate the potential link between thyroid disorders and gynecological cancers in adult females. The studies were categorized into two groups: group 1 examined the thyroid status of patients with various gynecological cancers, whereas group 2 consisted of case-control studies exploring the incidence of gynecological cancers in patients with thyroid disorders compared with controls.
Studies 2, 3, 5, 6, 7, 8, 9, 11, 12, 13, 14, 18, and 19 included data from 33,337 females diagnosed with various types of gynecological cancers, of whom 4,228 had thyroid disorders. They revealed that approximately 8% of females with gynecological cancers also have thyroid disorders. Hypothyroidism was predominant, accounting for 13% (95% CI, 10–17%) of females with gynecological cancers. This suggests a potential association between hypothyroidism and gynecological cancer, which is particularly evident in studies focusing on uterine cancer (12% in study 7 and 13% in study 14). In contrast, another study [16] reported a lower percentage of patients (5%).
Across studies comparing the prevalence of hypothyroidism and hyperthyroidism in females with gynecological cancers versus controls (studies 2, 7, 8, and 19), the proportional meta-analysis yielded an overall percentage for hypothyroidism of 14% (95% CI, 9–22%), whereas for hyperthyroidism, it was 3% (95% CI, 2–5%). The overall OR was 2.65 (P<0.05) (studies 9, 11, 12, and 13), indicating that women with uterine cancer and tumors have 2.65 times greater odds of developing hypothyroidism than those without them. However, for ovarian cancer in the study by Ness et al. [27], the OR was lower (0.92; P=0.6), indicating strong association of hyperthyroidism with ovarian cancer, which may be confirmed in the same study with an OR of 1.81 and a P-value of 0.02.
In contrast, for case-control studies (1, 4, 15, 16, and 17), the overall risk ratio was 1.12 with a P-value of 0.27, indicating a slight, non-significant increase in the risk of gynecological cancers among females with any thyroid disorder. However, when focusing on hypothyroidism, specifically in studies 1, 4, 15, and 17, the risk ratio increased to 1.3 (P<0.05), suggesting a greater risk compared to controls. In contrast, hyperthyroidism had a risk ratio of 0.87 (P=0.62), indicating non-significant decrease in the risk of gynecological cancers among females with hyperthyroidism. Particularly, for study 10, the risk ratio for ovarian cancer mortality in females with hyperthyroidism was 2.14 (P=0.03), whereas the risk ratio for ovarian cancer mortality in females with hypothyroidism showed a non-significant increase (RR, 1.35; P=0.26).
Therefore, this meta-analysis contributes to the growing body of evidence linking thyroid disorders with gynecological cancers, emphasizing the clinical significance of thyroid function assessment in these malignancies.
Despite these insights, this study had several limitations. The rare incidence of certain gynecological cancers, such as vulvar and vaginal cancers, has led to lack of studies and limited statistical power when collectively analyzing these specific cancer types among female genital tract cancers. Additionally, the number of included studies focusing on cancers other than uterine cancer, particularly ovarian cancer, was limited. Therefore, further research is necessary to provide more robust evidence regarding the association between the thyroid status and various gynecological cancers, particularly those that are less frequently studied. Future research should incorporate both mechanistic and experimental studies to advance our understanding. In vivo research could help elucidate the biological pathways linking thyroid disorders with gynecological cancers and provide more robust evidence of causality.
Hypothyroidism is notably associated with various gynecological cancers, suggesting a potential role in their pathogenesis. Conversely, hyperthyroidism is associated with an increased risk of ovarian cancer-related mortality. Further research is needed to clarify whether hyperthyroidism predisposes females to ovarian cancer and to assess the potential role of hypothyroidism in the pathogenicity of gynecological cancers.
References
1. Shahid MA, Ashraf MA, Sharma S. Physiology, thyroid hormone [Internet]. Treasure Island (FL): StatPearls Publishing;c2023. [cited 2023 Jun 5]. Available from: https://europepmc.org/article/NBK/nbk500006.
2. Tran TV, Kitahara CM, de Vathaire F, Boutron-Ruault MC, Journy N. Thyroid dysfunction and cancer incidence: a systematic review and meta-analysis. Endocr Relat Cancer. 2020; 27:245–59.

3. Leung JH, Wang SY, Leung HWC, Yu TS, Chan ALF. Hypothyroidism and hyperthyroidism related to gynecologic cancers: a nationwide population-based cohort study. Sci Rep. 2024; 14:1892.

4. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.

5. Almadrones-Cassidy LA. Gynecologic cancers. 1st ed. Pittsburgh: Oncology Nursing Society;2010.
6. Lee DY, Lee TS. Associations between metabolic syndrome and gynecologic cancer. Obstet Gynecol Sci. 2020; 63:215–24.

7. Kang JH, Kueck AS, Stevens R, Curhan G, De Vivo I, Rosner B, et al. A large cohort study of hypothyroidism and hyperthyroidism in relation to gynecologic cancers. Obstet Gynecol Int. 2013; 2013:743721.

8. Liao Y, Yang B. Correlation of hypothyroidism and endometrial carcinoma. Cancer Research on Prevention and Treatment. 2019; 46:452–5.
9. Elgebaly MM, Abdel-Hamed AR, Mesbah NM, Abo-Elmatty DM, Abouzid A, Abdelrazek MA. Hypothyroidism affect progression and worse outcomes of breast cancer but not ovarian cancer. J Immunoassay Immunochem. 2022; 43:288–98.

10. Shi XZ, Jin X, Xu P, Shen HM. Relationship between breast cancer and levels of serum thyroid hormones and antibodies: a meta-analysis. Asian Pac J Cancer Prev. 2014; 15:6643–7.

11. Du D, Li X. The relationship between thyroiditis and polycystic ovary syndrome: a meta-analysis. Int J Clin Exp Med. 2013; 6:880–9.
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–5.

13. Aga SS, Jaha R, Khan R, Junaydi D, Hakami AY, Khan MA, et al. Detailed demographics and the prevalence of comorbidities in ovarian cancer patients in Western Region of Saudi Arabia. J Nat Sci Med. 2022; 5:254–61.

14. Díez JJ, Iglesias P. Malignant neoplasms in people with hypothyroidism in Spain: a population-based analysis. PLoS One. 2022; 17:e0275568.

15. Li S, Li W, Sheng B, Zhu X. Relationship between thyroid disorders and uterine fibroids among reproductive-age women. Endocr J. 2021; 68:211–9.

16. Jha S, Singh A, Sinha HH, Bhadani P, Anant M, Agarwal M. Rate of premalignant and malignant endometrial lesion in “low-risk” premenopausal women with abnormal uterine bleeding undergoing endometrial biopsy. Obstet Gynecol Sci. 2021; 64:517–23.

17. Vilardo N, Dagum C, Arora A, Fazzari M, King K, Gressel G. Thyroid function and survival outcomes in women with uterine cancer. Gynecologic Oncology. 2021; 162:S309.

18. Almehmadi M, Alzahrani K, Salih MM, Alsharif A, Alsiwiehri N, Shafie A, et al. Assessment of thyroid gland function by evaluating of TSH, FT3 and FT4 hormones in untreated cancer patients. J Adv Pharm Educ Res. 2020; 10:37–42.
19. Journy NMY, Bernier MO, Doody MM, Alexander BH, Linet MS, Kitahara CM. Hyperthyroidism, hypothyroidism, and cause-specific mortality in a large cohort of women. Thyroid. 2017; 27:1001–10.

20. Kurnit KC, Ward KK, McHale MT, Saenz CC, Plaxe SC. Increased prevalence of comorbid conditions in women with uterine cancer. Gynecol Oncol. 2015; 138:731–4.

21. Ott J, Kurz C, Braun R, Promberger R, Seemann R, Vytiska-Binstorfer E, et al. Overt hypothyroidism is associated with the presence of uterine leiomyoma: a retrospective analysis. Eur J Obstet Gynecol Reprod Biol. 2014; 177:19–22.

22. Soleymani E, Ziari K, Rahmani O, Dadpay M, Taheri-Dolatabadi M, Alizadeh K, et al. Histopathological findings of endometrial specimens in abnormal uterine bleeding. Arch Gynecol Obstet. 2014; 289:845–9.

23. Seebacher V, Hofstetter G, Polterauer S, Reinthaller A, Grimm C, Schwameis R, et al. Does thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trial. Br J Cancer. 2013; 109:215–8.

24. Chen YK, Lin CL, Cheng FT, Sung FC, Kao CH. Cancer risk in patients with Hashimoto’s thyroiditis: a nationwide cohort study. Br J Cancer. 2013; 109:2496–501.

25. Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC, et al. Cancer risk in patients with Graves’ disease: a nationwide cohort study. Thyroid. 2013; 23:879–84.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download