Abstract
Although relatively rare, infections related to spinal pain interventions pose significant risks with an increase in the frequency of these procedures. This review investigates the incidence, risk factors, and management of infections following spinal pain interventions, such as epidural steroid injections, nucleoplasty, and facet joint injections. Most of the existing literature comprises case reports and retrospective studies with limited prospective research, owing to the nature of these infections. Our analysis revealed that while the overall infection rate is low, potential complications, such as epidural abscesses and spondylodiscitis, can be severe and life-threatening. The risk factors include advanced age, diabetes, immunosuppression, and multiple spinal procedures. Early diagnosis and timely intervention are critical to prevent long-term morbidity. These findings emphasize the importance of developing standardized diagnostic algorithms and treatment guidelines to support clinicians in managing these infections effectively. Future research should focus on large-scale studies to understand the impact of these infections better and refine clinical management strategies.
The increase in the frequency of spinal procedures, including epidural nerve blocks, to manage spinal pain has increased the risk of adverse events. In addition to epidural blocks, commonly performed spinal pain interventions include facet joint blocks, nerve root blocks, and procedures performed within the disc, such as nucleoplasty. While secondary infections resulting from these procedures are rare, they can lead to severe complications when they do occur [1,2]. Spinal infections have been reported in 1–2% of spinal injection cases, varying from moderate to severe diseases, including meningitis, epidural abscess, spondylitis, spondylodiscitis, and osteomyelitis. Acute infections are rare, affecting approximately 0.01–0.1% of spinal injection cases or between 1 in 10,000 and 1 in 1,000 procedures [3].
Consequently, understanding the prevention and prompt identification and management of infections related to spinal pain interventions are essential to provide adequate patient care. This review addresses the diagnosis of infections related to spinal pain interventions and the specific issues and their management.
A study conducted at a tertiary hospital in South Korea from January 2004 to December 2009 examined 116 patients diagnosed with and treated for infectious spondylitis [4]. Among them, 55% had undergone recent spinal procedures, while 18% developed infectious spondylitis following an epidural block. These numbers were derived from patients who developed spinal infections; thus, the incidence within the overall population of patients undergoing spinal interventions remains undetermined. In another retrospective, observational, and medicolegal study, precedents related to complications of epidural injections from January 1997 to August 2019 were analyzed using the database of the Supreme Court of Korea's judgment system [5]. Among the 49 malpractice cases included in the final analysis, the most common complication was infection (13 cases, 26.5%), followed by worsening pain (8 cases, 16.3%). Spinal infections resulting from the interventions could result in significant medical malpractice claims.
A recent study analyzed the 10-year national trend of single-shot epidural injections using the South Korean National Health Insurance Service sample cohort database [6]. The study also examined the incidence and risk factors associated with deep spinal infections following these procedures. New-onset deep spinal infections were defined as those arising within 90 days following the most recent outpatient single-shot epidural injection, necessitating hospitalization for at least one night and a treatment course of antibiotics lasting at least 4 weeks. The findings indicated that the number of outpatient single-shot epidural injections per 1,000 individuals increased from 40.8 in 2006 to 84.4 in 2015 in South Korea. Between 2007 and 2015, 501,509 injections were administered, resulting in the identification of 52 cases of deep spinal infection within 90 days after the procedure. This yields an infection rate of 0.01% per injection, which corroborates the findings of previous reports [3]. The risk factors included age ≥ 65 years, living in rural areas, having complicated diabetes, receiving three or more epidural injections in the previous 90 days, and recent use of immunosuppressants. This study showed that infections related to spinal pain interventions (single-shot epidural block) are rare, with an incidence of approximately 0.01% in South Korea. Furthermore, it proposed some risk factors related to infections during spinal pain interventions. In addition to spinal interventions, age in the 60s is a risk factor for overall infectious spondylitis, and the risk is 1.5–3.0 times higher in males than in females. Underlying conditions, such as diabetes, coronary artery disease, immunosuppression, alcoholism, liver cirrhosis, cancer, and chronic kidney disease, are also associated with increased risk [7-9].
We searched the Web of Science Core Collection database to investigate infections related to spinal pain interventions published until 2023 and indexed in the Science Citation Index Expanded and the Emerging Sources Citation Index. Our search strategy included topic searches for terms related to infection (infection or septic or abscess or osteomyelitis or discitis or spondylodiscitis or pyogenic or bacterial infection) and title searches for terms related to spinal interventions (spinal pain intervention or spinal procedure or epidural injection or nerve block or facet joint injection or spinal cord stimulator or radiofrequency ablation or spinal intervention or fluoroscopic guided or intradiscal injection or spinal anesthesia or pain management procedure).
The studies encompassed various infections related to spinal pain interventions, including epidural injections (N = 6) and spinal cord stimulators (N = 2), and followed diverse study designs, such as retrospective studies (N = 2), case reports (N = 9), and editorials (N = 1) (Table 1) [2,6,10-18]. Prospective studies are not feasible because of the nature of the infections associated with spinal pain interventions, and most available data are derived from retrospective studies or case reports. Thus, post-spinal procedure infections can develop because of various spinal interventions.
Prevalent infections associated with spinal pain interventions and surgeries include pyogenic spondylitis and spondylodiscitis, which arise from hematogenous dissemination, direct external inoculation, or contiguous tissue spread [19]. Hematogenous pyogenic spondylodiscitis predominantly affects the lumbar spine, followed by the thoracic and cervical spines, and it typically begins in the anterior or lateral part of the spine, which has a rich vascular supply. The arteries that supply the vertebrae originate from the vertebral, intercostal, lumbar, or sacral arteries and are situated on the anterolateral sides of the vertebral bodies. Small arterioles from this network bifurcate within the vertebral bodies and traverse the central nutritional foramen, exhibiting the most extensive distribution in the anterior subchondral region of the endplates, where infectious alterations typically commence [20].
Regarding direct external inoculation, iatrogenic inoculation typically occurs during surgeries, interventional or diagnostic procedures (e.g., lumbar puncture or discography), spinal pain interventions (e.g., epidural injection or nerve or facet block), vertebroplasty, kyphoplasty, or the use of indwelling catheters [21,22]. Infections related to spinal pain interventions primarily affect specific vertebral structures depending on the procedure used, with the posterior column of the spine being particularly affected [20]. This distinction is crucial for differentiating between hematogenous spread and secondary infection following iatrogenic procedures. Following a percutaneous selective spinal nerve root block, widespread inflammatory alterations may be noted along the needle trajectory, primarily within the back musculature. Inflammation may arise at injection sites, potentially extending into the paraspinal soft tissues and epidural space. Additionally, facet joint infections may occur because of direct inoculation during interventional procedures; severe infections within the facet joint may also be observed [17, 23].
Spinal infections are commonly characterized by the presence of certain red flags (Table 2) that have been identified as important signs in the analysis of the 16 guidelines related to spinal emergencies. Red flags in the spine are warning signals that may indicate serious spinal conditions that require prompt further evaluation and treatment. They primarily suggest the possibility of severe spinal disorders, such as infection, cancer, fractures, or neurological damage [24]. Among the red flags, the following factors are directly or indirectly associated with spinal infections: fever (mentioned in 12 guidelines), use of corticosteroids or immunosuppressant therapy (10 guidelines), and intravenous drug abuse (11 guidelines). Additionally, symptoms such as “pain worsening at night,” “intense nocturnal pain,” “pain at night and pain on resting,” “fever/chills,” and “bone tenderness over the lumbar spinous process” are commonly highlighted as important indicators. Particularly, there have been cases where secondary infections were detected after the procedure, despite the absence of fever or abnormal laboratory findings. Therefore, if a patient complains of severe back pain at the procedure site, it should be considered an important sign, and appropriate examinations and follow-up should be conducted based on the symptoms [13].
Secondary infection signs following spinal pain interventions generally manifest within 4 to 6 weeks [25,26]. However, this timeline may vary based on the patient's immune status, making it challenging to establish a precise definition. In another study, the average diagnosis time of pyogenic spondylitis, which arises following systemic infection or spinal procedures, was 27.3 ± 35.5 days. In contrast, tuberculous spondylitis had an average diagnosis time of 92.2 ± 70.9 days, indicating that variations in causative organisms may be significant [4].
Magnetic resonance imaging (MRI) is the gold standard method for diagnosing infections associated with spinal pain interventions [20]. MRI should be performed as soon as an infection is suspected because it has very high sensitivity and specificity for detecting early infections [27]. Although erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels are often elevated in infections, laboratory evaluations are not always reliable, making imaging particularly important.
The findings of pyogenic spondylitis and spondylodiscitis arising from hematogenous dissemination begin in the disc, soft tissue anterior to the disc, or epidural space. The initial indication of an infectious process may manifest as a modified marrow signal in a single vertebra [28]. The specific MRI findings include endplate cortical change, bone marrow signal change in an isolated vertebral body, and inflammatory change of paraspinal tissues and epidural space [20], and these findings develop progressively. In the early phase, the endplate cortical changes in the anterior vertebral body may appear hypointense on T1-weighted imaging (T1WI) or hyperintense on T2WI. This needs to be differentiated from Modic changes, as Modic Type 1 similarly shows the endplate cortical region, including bone marrow edema, as hypointense on T1WI and hyperintense on T2WI [29]. In the early stages, mild paravertebral soft tissue changes are commonly detected on contrast-enhanced images, and prevertebral and epidural inflammation can be observed alongside normal vertebral bodies. Over time, enhancement of the discs, vertebral bodies, and paravertebral soft tissues is often observed, although these signs may occasionally be absent. In most cases, epidural enhancement occurs anteriorly [30].
MRI findings of infections related to spinal pain interventions may show inflammatory changes that spread along the pathway of the procedure conducted. Inflammation typically begins at the skin and back muscles where the needle or instrument is inserted, injection sites, and around the nerve root and then spreads primarily into the paraspinal soft tissues and posterior epidural space [24,31]. However, the findings of pyogenic spondylitis and spondylodiscitis arising from hematogenous dissemination and the MRI findings of infections related to spinal pain interventions can be difficult to differentiate in the early stages. Consequently, it is crucial to assess these factors in conjunction with the patient's clinical symptoms as they progress.
Infections associated with hematogenous spread or spinal pain interventions can benefit from laboratory findings to assist in diagnosis. ESR and CRP are the most sensitive early screening tools for infections. CRP is a recognized sensitive laboratory marker that elevates within 6 h following the initiation of bacterial infection. Moreover, 90% of patients with spinal infections show elevated CRP levels, which can also be used as an indicator of the patient's response to infection treatment [32]. Meyer et al. [33] reported that CRP has 100% sensitivity and 95.8% specificity for predicting postoperative infections. The study by Mustard et al. [34] included 108 individuals, and a positive CRP response was defined as fulfillment of the following two criteria: (1) CRP > 80% of that on Day 2 on Days 3 and 4 (positive diagnosis by Day 4); and (2) an increase in the CRP level on two consecutive days after Day 4, with daily levels reaching 15 mg/l (positive diagnosis by Day 6). These CRP criteria showed 63% sensitivity, 82% specificity, and positive and negative predictive values of 68% and 78%, respectively. ESR is also a sensitive marker but shows low specificity and rises later as compared to CRP. In a prospective spine surgery study, the optimal cutoff value for elevated ESR to identify surgical site infections was > 51.5 mm/h on the 6th postoperative day, while that for CRP was > 5.94 mg/dl on the 3rd day and > 3.49 mg/dl on the 6th day [35]. In another prospective spine surgery study, the maximum mean peak ESR in the non-infection group occurred on the 4th postoperative day and normalized by the 14th day [36]. If spinal pain intervention-related infection arises, CRP and clinical symptoms can assist in its detection, and an elevated ESR after 6 days may confirm it.
When an infection is suspected, blood culture helps determine the pathogen and aids in diagnosis, thereby guiding the choice of appropriate antibiotics. In pyogenic spondylodiscitis cases, approximately 59% of positive blood cultures reveal the causative micro-organism [37], while others either have negative blood cultures or no organisms are detected, which requires careful interpretation of the test results. The most common causative organism is Staphylococcus aureus, followed by Staphylococcus epidermidis and Streptococcus spp., which together account for more than 50% of the cases [38]. These are skin commensals and are common causative agents of infections resulting from spinal pain interventions where instruments or needles are introduced from outside. In other studies, S. aureus is the predominant pathogenic bacterium, accounting for 30–80% of cases, followed by gram-negative bacilli, including Escherichia coli (approximately 25%), and Streptococcus and Enterococcus species. Additionally, Mycobacterium tuberculosis frequently occurs (approximately 60%) in individuals infected with the human immunodeficiency virus, whereas anaerobic agents can induce infections in cases of penetrating spinal injury. Nevertheless, in one-third of spinal infections, the causal factors remain unidentified [39]. A strategy for identifying and diagnosing infection-related spinal pain intervention is shown in Fig. 1.
Regardless of the cause, pyogenic spondylitis requires treatment with intravenous antibiotics. Due to the pain experienced before treatment, it often makes daily activities difficult, necessitating hospitalization. The use of antibiotics is the main treatment for pyogenic spondylitis, with 50–75% of patients showing improvement within 1 year without surgery [40,41]. Although the appropriate duration of antibiotic therapy has not been established, intravenous antibiotics are usually required for approximately 6 weeks to maintain adequate antibiotic concentration in the bone, as it takes approximately 6 weeks for vascularized soft tissue to cover the debrided bone and restore the blood supply [9]. If the ESR does not drop by at least two-thirds of its pre-treatment value or if the CRP level does not normalize, a re-evaluation and an extension of the treatment period might be required [9]. Several studies have reported that intravenous antibiotics are often switched to oral antibiotics after 6 weeks of treatment [42,43], and in some cases, antibiotics are administered for up to 3 months [44,45]. Immobilization is one of the key elements of a successful conservative treatment approach. When pain is severe and there is no risk of instability, immobilizing the affected area becomes essential. This strategy also helps avoid the need for extended bed rest. When the cervical spine is affected, immobilization can be achieved with a neck brace or halo-fixation device. When the thoracic or lumbar spine is affected, a thoracolumbar brace can help distribute the load to the unaffected joints and reduce the pressure on the injured vertebrae [39,42].
Neurological deficits and sepsis are the primary indications for surgery in patients with spinal infections. Additionally, conditions such as spinal instability due to extensive bone destruction, severe kyphosis, lesions within the spinal canal causing mass effects, and unexplained conditions associated with active tumors are indications for early surgical intervention [37]. Some authors suggest that even in the absence of neurological deficits, surgical treatment should be considered in cases of epidural abscesses, particularly in the cervical and thoracic regions [46]. The relative indications for surgery include uncontrolled pain or when conservative treatment is not feasible. Even when surgery is indicated because of neurological deficits, factors such as age and the presence of comorbidities can influence the decision to perform surgery. In another study [47] involving 45 older patients with pyogenic spondylitis, 42% of those presenting with paralysis during admission did not undergo surgery because of their poor overall condition. However, 73% of these patients showed improvement in paralysis with conservative treatment. When deciding between conservative and surgical treatments, the most important factors are the patient's neurological symptoms and overall condition. These considerations influence the clinician's decision to continue conservative treatment or proceed with or switch to surgical intervention, which can ultimately affect the patient's prognosis.
The exact incidence of infections related to spinal pain interventions has not been well established. Although these infections are less common than postoperative or systemic spinal infections, their significance increases as the number of spinal procedures increases. This review discusses the situations in which infections related to spinal pain interventions should be suspected, the diagnostic process, and appropriate testing methods. Furthermore, it provides an overview of the potential treatment options. We hope that algorithms and guidelines for the diagnosis and treatment of infections related to spinal pain interventions will be developed in the future.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Eun Joo Choi and Sang Cheol Yoon. Writing - review & editing: Eun Joo Choi and Sang Cheol Yoon. Conceptualization: Eun Joo Choi. Data curation: Sang Cheol Yoon. Methodology: Sang Cheol Yoon. Investigation: Eun Joo Choi and Sang Cheol Yoon. Supervision: Eun Joo Choi.
REFERENCES
1. Bicket MC, Chakravarthy K, Chang D, Cohen SP. Epidural steroid injections: an updated review on recent trends in safety and complications. Pain Manag. 2015; 5:129–46.

2. Hooten WM, Kinney MO, Huntoon MA. Epidural abscess and meningitis after epidural corticosteroid injection. Mayo Clin Proc. 2004; 79:682–6.

3. Windsor RE, Storm S, Sugar R. Prevention and management of complications resulting from common spinal injections. Pain Physician. 2003; 6:473–83.
4. Kim YI, Kim SE, Jang HC, Jung SI, Song SK, Park KH. Analysis of the clinical characteristics and prognostic factors of infectious spondylitis. Infect Chemother. 2011; 43:48–54.

5. Cho SI, Shin S, Jung H, Moon JY, Lee HJ. Analysis of judicial precedent cases regarding epidural injection in chronic pain management in Republic of Korea. Reg Anesth Pain Med. 2020; 45:337–43.

6. Lee CS, Park YJ, Moon JY, Kim YC. Deep spinal infection after outpatient epidural injections for pain: a retrospective sample cohort study using a claims database in South Korea. Anesthesiology. 2021; 134:925–36.

7. Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009; 39:10–7.

10. Shofwan S, Liem L, Janitra G, Basuki N, Rhatomy S. Discitis following radiofrequency nucleoplasty: a case report. Anesth Pain Med. 2020; 10:e110322.

11. Lee JW, Lee E, Lee GY, Kang Y, Ahn JM, Kang HS. Epidural steroid injection-related events requiring hospitalisation or emergency room visits among 52,935 procedures performed at a single centre. Eur Radiol. 2018; 28:418–27.

12. Wu B, He X, Peng BG. Pyogenic discitis with an epidural abscess after cervical analgesic discography: a case report. World J Clin Cases. 2020; 8:2318–24.

13. Lee SJ, Choi EJ, Nahm FS. Spondylodiscitis after cervical nucleoplasty without any abnormal laboratory findings. Korean J Pain. 2013; 26:181–5.

14. Chen F, Lü G, Kang Y, Ma Z, Lu C, Wang B, et al. Mucormycosis spondylodiscitis after lumbar disc puncture. Eur Spine J. 2006; 15:370–6.

15. Asakura Y. Just think about pyogenic spondylodiscitis before performing the epidural steroid injection for low back pain. Korean J Anesthesiol. 2018; 71:161–2.

16. Shinta Y, Hori M, Okada H. Pyogenic spondylitis four years after an injection into the paraspinal muscles. Cureus. 2024; 16:e64810.

17. Park MS, Moon SH, Hahn SB, Lee HM. Paraspinal abscess communicated with epidural abscess after extra-articular facet joint injection. Yonsei Med J. 2007; 48:711–4.

18. Okazaki K, Sasaki K, Matsuda S, Yuge I, Omiya K, Kido H, et al. Pyogenic arthritis of a lumbar facet joint. Am J Orthop (Belle Mead NJ). 2000; 29:222–4.
19. Lim S, Yoo YM, Kim KH. No more tears from surgical site infections in interventional pain management. Korean J Pain. 2023; 36:11–50.

20. Yeom JA, Lee IS, Suh HB, Song YS, Song JW. Magnetic resonance imaging findings of early spondylodiscitis: interpretive challenges and atypical findings. Korean J Radiol. 2016; 17:565–80.

21. Dullerud R, Nakstad PH. Side effects and complications of automated percutaneous lumbar nucleotomy. Neuroradiology. 1997; 39:282–5.

22. Guyer RD, Ohnmeiss DD, Mason SL, Shelokov AP. Complications of cervical discography: findings in a large series. J Spinal Disord. 1997; 10:95–101.
23. Weingarten TN, Hooten WH, Huntoon MA. Septic facet joint arthritis after a corticosteroid facet injection. Pain Med. 2006; 7:52–6.

24. Verhagen AP, Downie A, Popal N, Maher C, Koes BW. Red flags presented in current low back pain guidelines: a review. Eur Spine J. 2016; 25:2788–802.

25. Bavinzski G, Schoeggl A, Trattnig S, Standhardt H, Dietrich W, Reddy M, et al. Microsurgical management of postoperative disc space infection. Neurosurg Rev. 2003; 6:102–7.

26. Ozuna RM, Delamarter RB. Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Orthop Clin North Am. 1996; 27:87–94.

27. Raghavan M, Lazzeri E, Palestro CJ. Imaging of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012; 16:8–19.

29. Oztekin O, Calli C, Kitis O, Adibelli ZH, Eren CS, Apaydin M, et al. Reliability of diffusion weighted MR imaging in differentiating degenerative and infectious end plate changes. Radiol Oncol. 2010; 44:97–102.

30. Millot F, Bonnaire B, Clavel G, Deramond H, Fardellone P, Grados F. Hematogenous Staphylococcus aureus discitis in adults can start outside the vertebral body. Joint Bone Spine. 2010; 77:76–7.

31. Narváez J, Nolla JM, Narváez JA, Martinez-Carnicero L, De Lama E, Gómez-Vaquero C, et al. Spontaneous pyogenic facet joint infection. Semin Arthritis Rheum. 2006; 35:272–83.

32. Kang BU, Lee SH, Ahn Y, Choi WC, Choi YG. Surgical site infection in spinal surgery: detection and management based on serial C-reactive protein measurements. J Neurosurg Spine. 2010; 13:158–64.

33. Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. Acta Neurochir (Wien). 1995; 136:145–50.

34. Bohnen JM, Haseeb S, Kasina R. C-reactive protein levels predict postoperative septic complications. Arch Surg. 1987; 122:69–73.

35. Zheng S, Wang Z, Qin S, Chen JT. Usefulness of inflammatory markers and clinical manifestation for an earlier method to diagnosis surgical site infection after spinal surgery. Int Orthop. 2020; 44:2211–9.

36. Jönsson B, Söderholm R, Strömqvist B. Erythrocyte sedimentation rate after lumbar spine surgery. Spine (Phila Pa 1976). 1991; 16:1049–50.

37. Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008; 105:181–7.

39. Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013; 22:2787–99.

40. Khan IA, Vaccaro AR, Zlotolow DA. Management of vertebral diskitis and osteomyelitis. Orthopedics. 1999; 22:758–65.

41. Wirtz DC, Genius I, Wildberger JE, Adam G, Zilkens KW, Niethard FU. Diagnostic and therapeutic management of lumbar and thoracic spondylodiscitis--an evaluation of 59 cases. Arch Orthop Trauma Surg. 2000; 120:245–51.
42. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000; 25:1668–79.

43. Roblot F, Besnier JM, Juhel L, Vidal C, Ragot S, Bastides F. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum. 2007; 36:269–77.

44. Livorsi DJ, Daver NG, Atmar RL, Shelburne SA, White AC Jr, Musher DM. Outcomes of treatment for hematogenous Staphylococcus aureus vertebral osteomyelitis in the MRSA ERA. J Infect. 2008; 57:128–31.

Fig. 1.
Algorithm for diagnosing infections related to spinal pain interventions. MRI: magnetic resonance imaging, WBC: white blood cell count, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate.
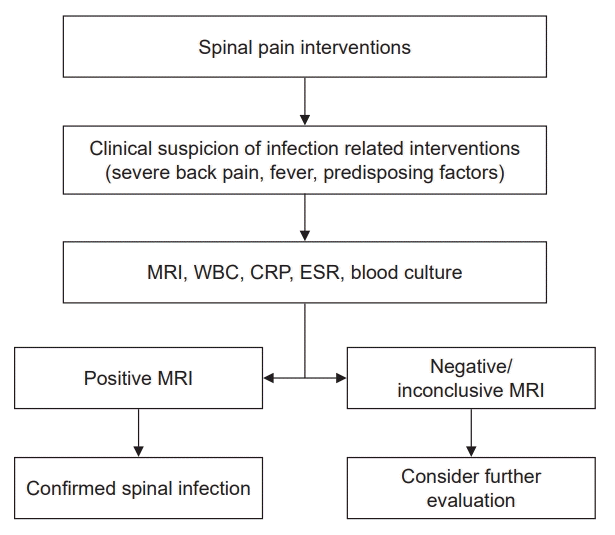
Table 1.
Previous Studies on Infections Related to Spinal Pain Interventions
| Author | Study | Study design | Type of block | Number of blocks | Number of infections | Remarks |
|---|---|---|---|---|---|---|
| Lee et al. [6] | Deep spinal infection after outpatient epidural injections for pain: a retrospective sample cohort study using a claims database in South Korea | Retrospective | Epidural/nerve block | 501509 | 52 | Infections resulted in hospitalization |
| Shofwan et al. [10] | Discitis following radiofrequency nucleoplasty: a case report | Case report | Nucleoplasty | 1 | 1 | Discitis after nucleoplasty; treated with antibiotics and rest |
| Hooten et al. [2] | Epidural abscess and meningitis after epidural corticosteroid injection | Case report | Epidural | 1 | 1 | Both patients developed meningitis post-injection |
| Lee et al [11] | Epidural steroid injection-related events requiring hospitalization or emergency room visits among 52,935 procedures performed at a single center | Retrospective | Epidural/spinal | 52935 | 1570 | Events included severe infections requiring hospitalization |
| Wu et al. [12] | Pyogenic discitis with an epidural abscess after cervical analgesic discography, a case report | Case report | Discography | 1 | 1 | Patient developed pyogenic discitis following discography |
| Lee et al. [13] | Spondylodiscitis after cervical nucleoplasty without any abnormal laboratory findings | Case report | Nucleoplasty | 1 | 1 | Spondylodiscitis post-nucleoplasty, despite normal laboratory findings |
| Chen et al. [14] | Mucormycosis spondylodiscitis after lumbar disc puncture | Case report | Lumbar Disc Puncture | 1 | 1 | A rare case of mucormycosis spondylodiscitis post-disc puncture; treated with antifungal therapy |
| Asakura [15] | Just think about pyogenic spondylodiscitis before performing the epidural steroid injection | Editorial | Epidural | - | 26 | An editorial stressing caution in performing epidural steroid injections to avoid spondylodiscitis |
| Shinta et al. [16] | Pyogenic spondylitis four years after an injection into the paraspinal muscles | Case report | Paraspinal injection | 1 | 1 | Pyogenic spondylitis developed 4 years after a paraspinal injection |
| Park et al. [17] | Paraspinal abscess communicated with epidural abscess after extra-articular facet injection | Case report | Facet joint injection | 1 | 1 | Paraspinal abscess developed and communicated with an epidural abscess after facet joint injection |
| Okazaki et al. [18] | Pyogenic arthritis of a lumbar facet joint | Case report | Facet joint injection | 1 | 1 | Pyogenic arthritis developed after lumbar facet joint injection |
| Weingarten et al. [23] | Septic facet joint arthritis after a corticosteroid facet injection | Case report | Facet joint injection | 1 | 1 | Septic arthritis noted post-facet joint corticosteroid injection |
Table 2.
Red Flags in Spinal Infections




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download