1. Altemir FH. The submental route for endotracheal intubation. A new technique. J Maxillofac Surg. 1986; 14:64–5.
2. Eisemann B, Eisemann M, Rizvi M, Urata MM, Lypka MA. Defining the role for submental intubation. J Clin Anesth. 2014; 26:238–42.

3. Altemir FH, Montero SH, Pena MM. About submental intubation. Anaesthesia. 2003; 58:496–7.

4. Jacob DD, Tuncer FB, Kashan DL, Gurunluoglu R. Clinical anatomy of submental intubation: A review of the indications, technique, and a modified approach. Ann Plast Surg. 2020; 84:232–7.
5. Goh EZ, Loh NHW, Loh JSP. Submental intubation in oral and maxillofacial surgery: A systematic review 1986-2018. Br J Oral Maxillofac Surg. 2020; 58:43–50.

6. Green JD, Moore UJ. A modification of sub-mental intubation. Br J Anaesth. 1996; 77:789–91.

7. Lim D, Ma BC, Parumo R, Shanmuhasuntharam P. Thirty years of submental intubation: A review. Int J Oral Maxillofac Surg. 2018; 47:1161–5.

8. MacInnis E, Baig M. A modified submental approach for oral endotracheal intubation. Int J Oral Maxillofac Surg. 1999; 28:344–6.

9. Altemir FH, Montero SH. The submental route revisited using the laryngeal mask airway: A technical note. J Craniomaxillofac Surg. 2000; 28:343–4.

10. Nwoku AL, Al-Balawi SA, Al-Zahrani SA. A modified method of submental oroendotracheal intubation. Saudi Med J. 2002; 23:73–6.
11. Hernandez Altemir F, Hernandez Montero S, Moros Pena M. Combitube SA through submental route. A technical innovation. J Craniomaxillofac Surg. 2003; 31:257–9.

12. Lim HK, Kim IK, Han JU, Kim TJ, Lee CS, Song JH, et al. Modified submental orotracheal intubation using the blue cap on the end of the thoracic catheter. Yonsei Med J. 2003; 44:919–22.

13. Yoon KB, Choi BH, Chang HS, Lim HK. Management of detachment of pilot balloon during intraoral repositioning of the submental endotracheal tube. Yonsei Med J. 2004; 45:748–50.

14. Kim KJ, Lee JS, Kim HJ, Ha JY, Park H, Han DW. Submental intubation with reinforced tube for intubating laryngeal mask airway. Yonsei Med J. 2005; 46:571–4.

15. Taglialatela Scafati C, Maio G, Aliberti F, Taglialatela Scafati S, Grimaldi PL. Submento-submandibular intubation: Is the subperiosteal passage essential? Experience in 107 consecutive cases. Br J Oral Maxillofac Surg. 2006; 44:12–4.
16. Nyárády Z, Sári F, Olasz L, Nyárády J. Submental endotracheal intubation in concurrent orthognathic surgery: A technical note. J Craniomaxillofac Surg. 2006; 34:362–5.

17. Biswas BK, Joshi S, Bhattacharyya P, Gupta PK, Baniwal S. Percutaneous dilational tracheostomy kit: An aid to submental intubation. Anesth Analg. 2006; 103:1055.

18. Lima SM Jr, Asprino L, Moreira RW, De Moraes M. A retrospective analysis of submental intubation in maxillofacial trauma patients. J Oral Maxillofac Surg. 2011; 69:2001–5.

19. Saheb SM, Nath VN, Kumar KP, Padmaja PP. A novel method using Seldinger's technique for submental intubation in major craniomaxillofacial fractures: A case series. Indian J Anaesth. 2014; 58:48–50.

20. Kita R, Kikuta T, Takahashi M, Ootani T, Takaoka M, Matsuda M, et al. Efficacy and complications of submental tracheal intubation compared with tracheostomy in maxillofacial trauma patients. J Oral Sci. 2016; 58:23–8.

21. Ujam A, Perry M. Minimally traumatic submental intubation: A novel dilational technique. Eur J Trauma Emerg Surg. 2017; 43:359–62.

22. Oshima N, Shiraishi T, Kawauchi T, Oba J, Sato D, Fujiki M, et al. A simple and reliable submental intubation technique for maxillofacial fractures. J Craniofac Surg. 2018; 29:1952–5.

23. Jung I, Yoo BH, Ju JY, Choi S, Yon JH, Kim KM, et al. Novel alternative for submental intubation: a case report. Anesth Pain Med (Seoul). 2020; 15:247–50.
24. Yun HJ, Rhee SH, Park JY, Chae YS, Han JH, Ryoo SH, et al. A novel technique of submandibular intubation with a camera cable drape: A case report. J Dent Anesth Pain Med. 2020; 20:155–60.

25. Silveira RL, Costa SM, Amaral MBF. New device for submental endotracheal intubation. J Craniofac Surg. 2020; 31:562–3.

26. Jeon YG, Lee C, Hong D, Jin Y, Lim HK. Modified submental intubation techniques for maxillofacial surgery - a report of five cases. Anesth Pain Med (Seoul). 2022; 17:331–7.
27. De Souza AAB, Araújo SCS, Martins GH, De Jesus AO, Amaral MBF, Silveira RL. New device for submental endotracheal intubation: A prospective cohort study. J Oral Maxillofac Surg. 2022; 80:1927–42.

28. Bihani P, Paliwal N, Jaju R, Chattopadhyay C, Chhabra A. Ultrasound-guided Seldinger technique-assisted submental intubation for panfacial trauma-walking off the beaten path. Saudi J Anaesth. 2023; 17:349–52.

29. Barik AK, Jain K, Bhatia N, Kumar RK. Use of Griggs forceps for submental intubation: A modification of Seldinger's technique. Indian J Anaesth. 2024; 68:310–1.

30. Troise S, Committeri U, Barone S, Palumbo D, D'Auria D, Arena A, et al. Submental intubation in complex maxillofacial trauma: Pilot balloon protection. J Craniomaxillofac Surg. 2024; 52:212–21.

31. Kiran M, Padala S, Preeti P, Seema S, Kuttan KA. An innovative method of pilot balloon capping for submental intubation. J Dent Anesth Pain Med. 2024; 24:139–41.

32. Oda A, Yoshida M, Imamura S, Takahashi T, Oue K, Doi M, et al. Anesthetic management of a patient with sturge-weber syndrome in sagittal split ramus osteotomy surgery. Clin Case Rep. 2024; 12:e8747.

33. SPS AR, Bhatia N, Jain K, Gupta T, Hazarika A. A prospective, randomized comparison of the classical altemir's method with the newer Seldinger's technique of submental intubation. Anesth Analg. 2023; 137:638–47.
34. Das S, Das TP, Ghosh PS. Submental intubation: A journey over the last 25 years. J Anaesthesiol Clin Pharmacol. 2012; 28:291–303.

35. Daniels JS, Albakry I, Braimah RO, Samara MI, Albalasi RA, Begum F, et al. Experience with airway management and sequencing of repair of panfacial fractures: A single tertiary healthcare appraisal in najran, kingdom of saudi arabia - a retrospective study. Ann Maxillofac Surg. 2020; 10:402–8.

36. Rodrigues WC, De Melo WM, De Almeida RS, Pardo-Kaba SC, Sonoda CK, Shinohara EH. Submental intubation in cases of panfacial fractures: A retrospective study. Anesth Prog. 2017; 64:153–61.

37. Banerjee PK, Jain A, Behera B. Submandibular intubation as an alternative for intra-operative airway management in maxillofacial fractures - our institutional experience. Indian J Anaesth. 2016; 60:573–7.

38. Hassanein AG, Abdel Mabood AM. Can submandibular tracheal intubation be an alternative to tracheotomy during surgery for major maxillofacial fractures? J Oral Maxillofac Surg. 2017; 75:508.e1–7.

39. Vellone V, De Tomaso S, Marianetti TM, Sabelli V, Ramieri V. Use of submental intubation in the full-face makeover (orthognathic surgery, facial prosthesis combined with rhinoplasty). J Craniofac Surg. 2023; 34:723–7.

40. Williams KD, Tariq M, Acharekar MV, Guerrero Saldivia SE, Unnikrishnan S, Chavarria YY, et al. Submental intubation in maxillofacial procedures: A more desired approach than nasotracheal intubation and tracheostomy. Cureus. 2022; 14:e27475.

41. Jundt JS, Cattano D, Hagberg CA, Wilson JW. Submental intubation: A literature review. Int J Oral Maxillofac Surg. 2012; 41:46–54.

42. Anwer HMF, Zeitoun IM, Shehata EAA. Submandibular approach for tracheal intubation in patients with panfacial fractures. Br J Anaesth. 2007; 98:835–40.

43. Hsieh YL, Hsu PC, Chen CH, Kao MC. Submental intubation in patients with complex facial bone fractures. Asian J Anesthesiol. 2021; 59:174–5.
44. Caron G, Paquin R, Lessard MR, Trépanier CA, Landry PE. Submental endotracheal intubation: An alternative to tracheotomy in patients with midfacial and panfacial fractures. J Trauma. 2000; 48:235–40.

45. Emara TA, El-Anwar MW, Omara TA, Anany A, Elawa IA, Rabea MM. Submental intubation versus tracheostomy in maxillofacial fractures. Oral Maxillofac Surg. 2019; 23:337–41.

46. Schütz P, Hamed HH. Submental intubation versus tracheostomy in maxillofacial trauma patients. J Oral Maxillofac Surg. 2008; 66:1404–9.

47. Panchamia JK, Hill D, Sharaf B, Amundson AW. Submental approach to intubation for craniomaxillofacial surgery. Can J Anaesth. 2021; 68:727–8.

48. Bhatia N, Gupta T, Patro S, Aswin Ram SPS, Valerian DQ, Jain K. Injury to the submandibular duct and secondary fibrosis causing sialocele: An unusual complication of submental intubation. J Maxillofac Oral Surg. 2024; 23:129–31.

49. Mishra R, Yadav D, Tripathi S, Kandel L, Baral PP, Shubham S, et al. Submental intubations in panfacial fractures. Clin Cosmet Investig Dent. 2020; 12:41–8.
50. Pitak-Arnnop P, Tangmanee C, Subbalekha K, Sirintawat N, Urwannachotima N, Auychai P, et al. Factors associated with complications of submental intubation in 339 patients with facial fractures: A German retrospective cohort study. J Stomatol Oral Maxillofac Surg. 2023; 124:101332.

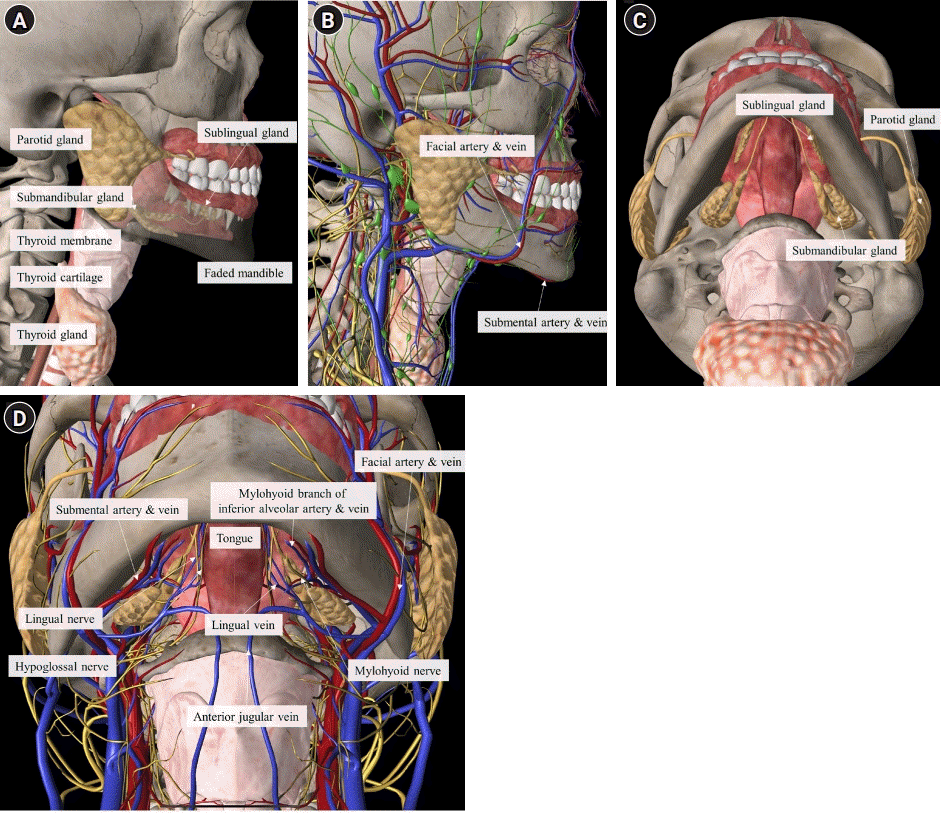
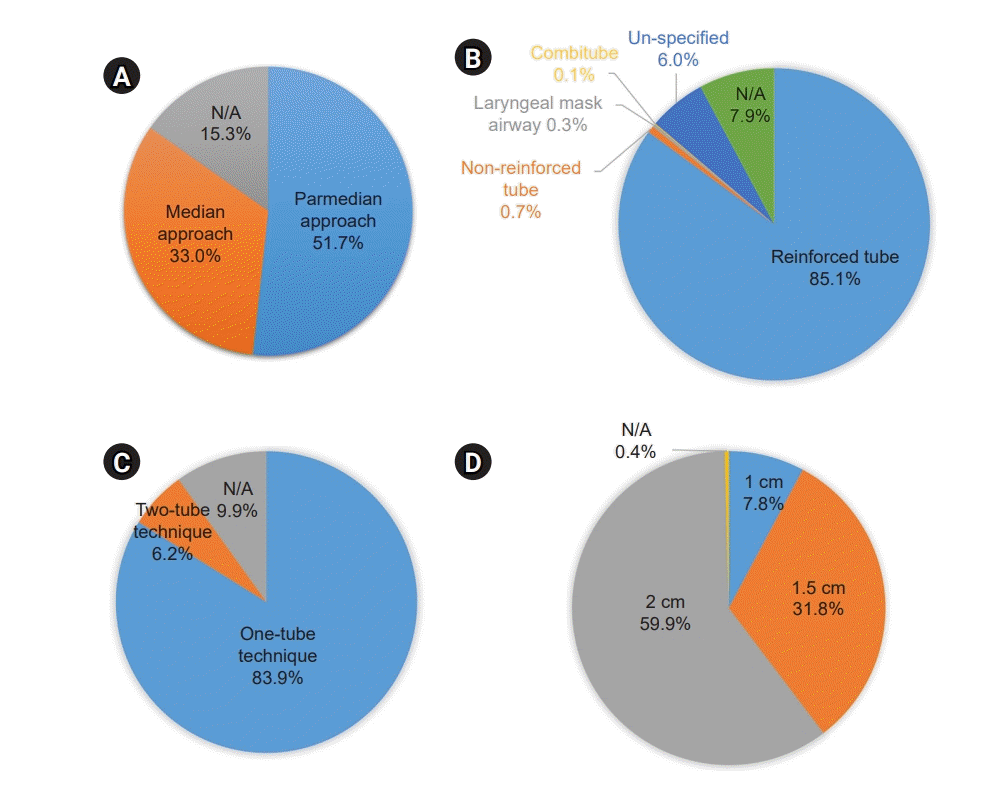
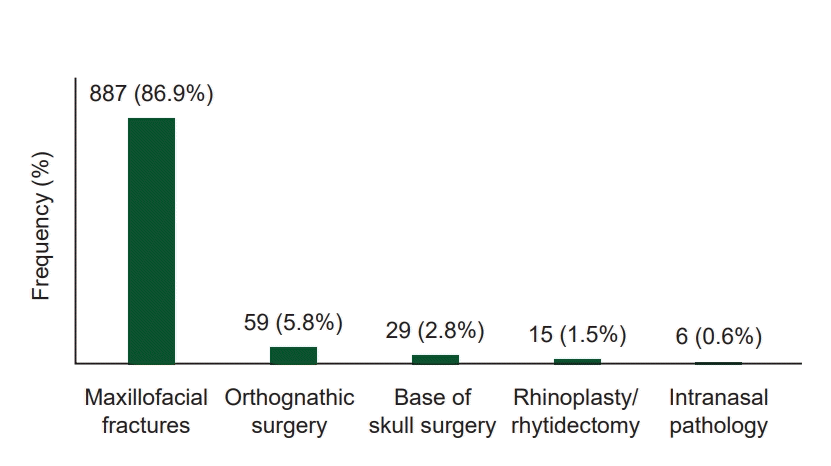




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download