Abstract
This is the first report of the successive development and rupture of blister-like anterior communicating artery (ACoA) aneurysms at mirror locations with a short interval. A 49-year-old man presented with an angiogram-negative subarachnoid hemorrhage with significant basal frontal interhemispheric blood. Surgical exploration revealed a blister-like aneurysm on the left side of the superior wall of the ACoA, which was treated using a microsuturing technique. On the 18th day after the initial subarachnoid hemorrhage, the second operation due to another angiogram-negative hemorrhage revealed a de novo blister-like aneurysm with a small blood clot on the posterosuperior wall of the ACoA close to the right A1/A2 junction. The rupture point and ACoA on the right side were occluded using an aneurysm clip. Follow-up digital subtraction angiogram at 4 years and computed tomography angiogram at 14 years after the surgery showed no recurrence or associated abnormality.
Blood blister-like aneurysms were originally reported as aneurysms with a fragile wide neck arising from the dorsal wall of the supraclinoid internal carotid artery (ICA) by Nakagawa et al. [15] in 1986. Histological characteristics of blood blister-like aneurysms include focal wall defects covered with a clot and fibrous tissue. According to an autopsy study of a blood blister-like aneurysm on the dorsal wall of the ICA by Ishikawa et al. [8], the arterial wall defect had no an internal elastic lamina or media and was covered with a thin adventitia and fibrinous tissue.
Although blood blister-like aneurysms have already been identified in the intracranial ICA [1,8,16,17], Andaluz and Zuccarello [3] reported the first case series and characterized the blood blister-like aneurysms arising from the anterior communicating artery (ACoA) in 2008. These blister-like ACoA aneurysms were named based on the shape of the aneurysmal bulge, which is extremely fragile and thin walled. At the time of surgical dissection, these lesions sometimes burst, leaving a hole in the dorsal side of the ACoA. Yet, blister-like ACoA aneurysms are very rare with only 10 previously reported cases presenting with a subarachnoid hemorrhage [2,3,13,18,20].
Here, we present the first known reported case of the successive development and rupture of two blister-like ACoA aneurysms at mirror locations. We include possible causes of the angiogram-negative non-perimesencephalic subarachnoid hemorrhage, rapid development and rupture of the blister-like ACoA aneurysm, available surgical techniques, and the long-term results of the surgical treatment, all of which have important clinical significance.
A 49-year-old man presented with a sudden bursting headache without mental deterioration. An immediate computed tomography (CT) scan revealed a diffuse, thin subarachnoid hemorrhage in the basal cisterns. A more pronounced collection of blood was noted in the inferior part of the frontal interhemispheric fissure, suggesting that the source of the hemorrhage was from a ruptured ACoA aneurysm (Fig. 1A). However, the digital subtraction angiogram (DSA) and computed tomography angiogram (CTA) revealed a small aneurysm at the bifurcation of the right middle cerebral artery (MCA) and no lesion in the ACoA region (Fig. 1B and C).
The patient underwent surgery 4 days after the subarachnoid hemorrhage to clip the MCA aneurysm and explore the ACoA region. A pterional craniotomy exposed a small MCA aneurysm in an unruptured state, which was clipped (Fig. 2A). Subsequent exploration of the ACoA region revealed a small blood clot, which was attached to the superior wall of an ACoA (Fig. 2B). Careful removal of the blood clot left a hole in the superior wall of the ACoA on the left side (Fig. 2C). There was no aneurysmal neck to clip directly. The presence of a perforator on the posterior wall of the ACoA adjacent to the hole, blister-like aneurysm, required direct repair of the rupture point instead of ACoA trapping. The hole of the ACoA was repaired using a microsuturing technique after temporary clipping of the bilateral A1 and A2 segments of the ACA. Three stitches with a 9-0 monofilament closed the hole satisfactorily, and the temporary clipping time was 15 minutes (Fig. 2D). The patient awoke from the surgery with a clear mentality and no neurological deficits. Postoperative angiography showed no evidence of stenosis or abnormality of the ACoA.
On the 18th day after the initial subarachnoid hemorrhage, the patient suddenly deteriorated to a stuporous mentality. A CT scan showed an intracerebral hemorrhage in the right frontal base starting from the ACoA region and intraventricular hemorrhage in the whole ventricular system (Fig. 3A), yet DSA did not reveal any problem in the intracranial vessels including the ACoA region (Fig. 3B).
The patient was immediately taken to surgery to explore the ACoA region. No abnormality was found in relation to the treated blister-like aneurysm of the ACoA on the left side, as the sutures were still tight and closing the hole. However, a de novo blister-like aneurysm with a small blood clot on the posterosuperior wall of the ACoA close to the right A1/A2 junction was found (Fig. 4A). As the location of the lesion hindered any microsuturing technique. The de novo blister-like aneurysm was treated using an angled fenestrated clip that was placed through the A1 segment with the clip blades occluding the rupture point and ACoA as there was no ACoA perforator adjacent to the lesion (Fig. 4B). The ACoA stump on the left side with the associated perforator was preserved and microvascular Doppler sonography verified normal perfusion of the bilateral A2 segments.
The patient improved to a clear mentality and his recovery was uneventful after discharge. A follow-up DSA at 4 years and CTA at 14 years after the surgery showed no interval change with an occluded ACoA on the right side and preserved ACoA stump on the left side, suggesting an intact ACoA perforator (Fig. 5). No evidence of aneurysmal recurrence was noticed in the ACoA and right MCA.
This case report highlights the presentation as an angiogram-negative subarachnoid hemorrhage, the rapid development and rupture of a de novo blister-like ACoA aneurysm, the available surgical techniques, and long-term results of the surgical treatment. Blood blister-like aneurysms in the ICA are already well known to neurosurgeons and characterized by a small fragile bulge at non-branching sites from the dorsomedial wall of the intracranial ICA [1,8,16,17]. These aneurysms only consist of adventitia and subadventitial fibrinous tissue at the site of a focal defect of the intima and media without any collagenous tissue that is normally seen with regular saccular aneurysms [8]. A focal wall defect in the ICA can sometimes be covered by a blood clot after a lesion rupture and subarachnoid hemorrhage.
Blood blister-like aneurysms have also recently been identified in the ACoA. Following the initial report of five cases by Andaluz and Zuccarello [3], seven other reports have since been published, as summarized in Table 1 [2,13,14,18-20,22]. A small fragile bulge was invariably observed in the horizontal portion of the ACoA, sometimes abutting the A2/ACoA junction. In three reports, seven cases of ruptured blister-like aneurysms were confirmed at surgery and treated [2,3,14]. In three other reports, three patients presented with a subarachnoid hemorrhage in the basal frontal interhemispheric fissure and a presumptive diagnosis of a blister-like aneurysm was made based on the angiographic findings. They were then treated endovascularly using a single stent or flow-diverter stent [13,18,20]. Plus, in two cases, a blister-like aneurysm was found in an unruptured state during surgery for a ruptured accessory ACA aneurysm and craniopharyngioma, respectively [19,22]. A very small, thin-walled bulge was found in the ACoA and regarded as a blister-like aneurysm.
Angiographically occult cerebral aneurysms mean aneurysms discovered during exploratory surgery in spite of several negative angiograms after a spontaneous subarachnoid hemorrhage. Very small saccular ACoA aneurysms with intrasaccular thrombosis are the most commonly reported angiographically occult aneurysms [4,6,9,25]. Yet, among the 11 reported cases of ruptured blister-like ACoA aneurysms, including the present case, the initial angiogram was negative in eight cases (72.7%). Thus, a blister-like ACoA aneurysm may also be an important type of angiographically occult cerebral aneurysm. A high index of suspicion is required for an angiogram-negative subarachnoid hemorrhage with significant basal frontal interhemispheric blood.
The pathogenesis of blister-like ACoA aneurysms is totally unknown. In two previous case reports, a very small thin-walled bulge in the ACoA was regarded as an unruptured blister-like ACoA aneurysm [19,22]. While the development of a blister-like ACoA aneurysm could be considered as slow, the present case showed the de novo development and rupture of a blister-like ACoA aneurysm with a short 3-week interval, possibly attributable to arterial dissection.
Intracranial aneurysms can develop de novo in previously healthy individuals or after successful treatment of another aneurysm. The time interval between the first episode of a subarachnoid hemorrhage and the second due to the rupture of a de novo aneurysm can be quite extended, 1 to 16 years [5,7,10,12,21]. Hypertension, a history of smoking, multiple and familial aneurysms, and hemodynamic changes after occlusion of the major cerebral vessels are known to be more frequently associated with the de novo development of aneurysms [5,11,21]. The development of a de novo aneurysm seems pursuant to chronic hemodynamic stress on a defect of the arterial wall that is congenital in origin.
Notwithstanding, the early development of a de novo aneurysm within a few months after a subarachnoid hemorrhage is extremely rare. To the author’s knowledge, there has only been one other report of an early de novo saccular aneurysm in the proximal A2 segment of the ACA producing another subarachnoid hemorrhage 30 days after the surgical clipping of an anterior choroidal artery aneurysm [24]. Therefore, the present case is the earliest development of a de novo aneurysm, where this very early development would seem to reflect a dissection-like presentation of a blister-like aneurysm rather than progression of a small saccular aneurysm due to chronic hemodynamic stress.
While common saccular ACoA aneurysms can be managed safely and adequately by placing an aneurysm clip across the neck of the aneurysm, repair of a blister-like ACoA aneurysm or hole in the ACoA developed by avulsion of the aneurysm neck can be challenging. Andaluz and Zuccarello [3] suggested application of a straight fenestrated clip that included the anterior cerebral artery-ACoA junction in the fenestration. However, exact clip application can be hindered due to the location of the lesion close to the A1/A2 junction and short length of the ACoA. Therefore, in the present case, the first blister-like ACoA aneurysm was repaired using a microsuturing technique, thereby avoiding any trapping of the ACoA and preserving the ACoA perforators adjacent to the lesion [23,26]. However, in the second surgery, microsuturing could not be applied, as the lesion was located on the posterosuperior wall of the ACoA close to the right A1/A2 junction and the brain was swollen due to acute repeated bleeding.
Although blister-like ACoA aneurysms seem to be challenging as regards their diagnosis and treatment, all previous reports of blister-like ACoA aneurysms have presented successful treatment results following both surgical and endovascular treatments. Yet, such excellent results may be attributable to a publication bias, where positive or favorable results tend to be preferentially reported by authors and published by journals. Moreover, the treatment follow-up periods in the previous reports are all short, between 1 to 5 months, whereas the current report has the longest follow-up ever published, DSA after 4 years and CTA after 14 years.
This is the first report of the successive development and rupture of blister-like ACoA aneurysms at mirror locations. A high index of suspicion is required for an angiogram-negative subarachnoid hemorrhage with significant basal frontal interhemispheric blood. The rapid development and rupture of a de novo blister-like ACoA aneurysm on the right side 18 days after the rupture of the first blister-like ACoA aneurysm on the left side was a dissection-like presentation of a blister-like aneurysm rather than the progression of a small saccular aneurysm due to chronic hemodynamic stress. A microsuturing technique is an effective and durable treatment option of a blister-like ACoA aneurysm, while focal ACoA occlusion is available in cases of no A1 segment hypoplasia and no associated ACoA perforator.
Notes
Conflicts of interest
Jaechan Park has been editorial board of JKNS since May 2020. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
References
1. Abe M, Tabuchi K, Yokoyama H, Uchino A. Blood blisterlike aneurysms of the internal carotid artery. J Neurosurg. 89:419–424. 1998.

2. Abiko T, Saito K, Murase M, Tomita H. Blister-like aneurysm originating from the anterior communicating artery:a case report. No Shinkei Geka. 46:207–212. 2018.
3. Andaluz N, Zuccarello M. Blister-like aneurysms of the anterior communicating artery: a retrospective review of diagnosis and treatment in five patients. Neurosurgery. 62:807–811. discussion 811. 2008.
4. Bradac GB, Bergui M, Ferrio MF, Fontanella M, Stura G. False-negative angiograms in subarachnoid haemorrhage due to intracranial aneurysms. Neuroradiology. 39:772–776. 1997.

5. Briganti F, Cirillo S, Caranci F, Esposito F, Maiuri F. Development of “de novo” aneurysms following endovascular procedures. Neuroradiology. 4:604–609. 2002.

6. Di Lorenzo N, Guidetti G. Anterior communicating aneurysm missed at angiography: report of two cases treated surgically. Neurosurgery. 23:494–499. 1988.

7. Graf CJ, Hamby WB. Report of a case of cerebral aneurysm in an adult developing apparently de novo. J Neurol Neurosurg Psychiatry. 27:153–156. 1964.

8. Ishikawa T, Nakamura N, Houkin K, Nomura M. Pathological consideration of a “blister-like” aneurysm at the superior wall of the internal carotid artery: case report. Neurosurgery. 40:403–405. discussion 405-406. 1997.

9. Jafar JJ, Weiner HL. Surgery for angiographically occult cerebral aneurysms. J Neurosurg. 79:674–679. 1993.

10. Kim DH, Jung JY, Lee JW, Huh SK, Lee KC. A clinical analysis of twelve cases of ruptured cerebral de novo aneurysms. Yonsei Med J. 48:30–34. 2007.

11. Leblanc R. De novo formation of familial cerebral aneurysms: case report. Neurosurgery. 44:871–876. discussion 876-877. 1999.

12. Maiuri F, Spaziante R, Iaconetta G, Signorelli F, Cirillo S, Di Salle F. ‘De novo’ aneurysm formation: report of two cases. Clin Neurol Neurosurg. 97:233–238. 1995.

13. Miyashita K, Nambu K, Shimizu Y, Tohma Y. Blister-like aneurysm of the anterior communicating artery treated with only low-profile visualized intraluminal support junior stent. Surg Neurol Int. 12:564. 2021.

14. Morris TC, Brophy BP. Blister-like aneurysm of the anterior communicating artery. J Clin Neurosci. 16:1098–1100. 2009.

15. Nakagawa F, Kobayashi S, Takemae T, Sugita K. Aneurysms protruding from the dorsal wall of the internal carotid artery. J Neurosurg. 65:303–308. 1986.

16. Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the surpaclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. 47:578–583. discussion 583-586. 2000.

17. Ohkuma H, Nakano T, Manabe H, Suzuki S. Subarachnoid hemorrhage caused by a dissecting aneurysm of the internal carotid artery. J Neurosurg. 97:576–583. 2002.

18. Peschillo S, Cannizzaro D, Missori P, Colonnese C, Santodirocco A, Santoro A, et al. Reconstructive endovascular treatment of a ruptured blood blister-like aneurysm of anterior communicating artery. J Neurosurg Sci. 61:438–441. 2017.

19. Qian H, Wang L, Brooks KS, Zhao X, Liu F, Sun Y, et al. Intraoperative finding of an anterior communicating artery blister-like aneurysm during a primary craniopharyngioma resection: accidental or incidental? World Neurosurg. 127:514–517. 2019.

20. Rouchaud A, Saleme S, Gory B, Ayoub D, Mounayer C. Endovascular exclusion of the anterior communicating artery with flow-diverter stents as an emergency treatment for blister-like intracranial aneurysms. A case report. Interv Neuroradiol. 19:471–478. 2013.

21. Sakaki T, Tominaga M, Miyamoto K, Tsunoda S, Hiasa Y. Clinical studies of de novo aneurysms. Neurosurgery. 32:512–516. discussion 516-517. 1993.

22. Seo DH, Lee WC, Choe IS, Park SC, Ha YS. Ruptured and unruptured aneurysms of the accessory anterior cerebral artery combined with a blood blister-like aneurysm of the anterior communicating artery. Neurol India. 57:85–87. 2009.

23. Serizawa T, Saeki N, Yamaura A. Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery. 40:1211–1216. discussion 1216-1218. 1997.

24. Sim JH, Kim SC, Kim MS. Early development and rupture of de novo aneurysm--case report. Neurol Med Chir (Tokyo). 42:334–337. 2002.
Fig. 1.
Initial radiological images on admission. A : Computed tomography scan showing a diffuse subarachnoid hemorrhage in the basal cisterns. The more pronounced collection of blood (arrow) in the inferior part of the frontal interhemispheric cistern suggests a hemorrhage from a ruptured ACoA aneurysm. B : CTA showing a small aneurysm (arrow) at the bifurcation of the right middle cerebral artery. C : CTA showing no lesion in the ACoA (arrow). ACoA : anterior communicating artery, CTA : computed tomography angiogram.
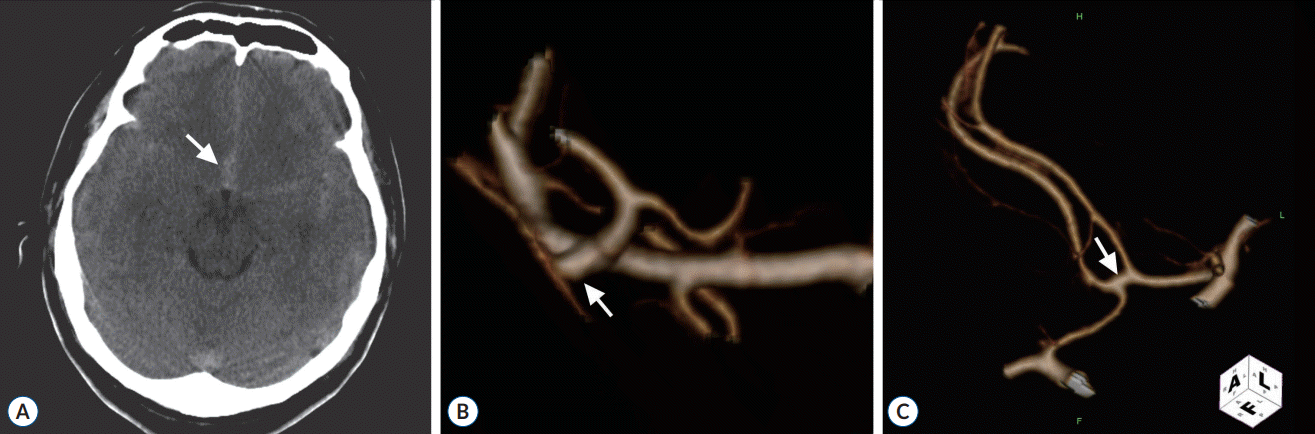
Fig. 2.
Intraoperative photographs of the first pterional craniotomy. A : Small unruptured aneurysm (arrow) at the bifurcation of the right middle cerebral artery. B : Small blood clot (double arrows) attached to the superior wall of the ACoA (arrow). C : Arterial hole (arrow) in the superior wall of the ACoA on the left side, which was exposed after careful removal of the blood clot. D : Three stitches (arrow) using 9-0 monofilament closing the arterial hole in the ACoA. ACoA : anterior communicating artery.
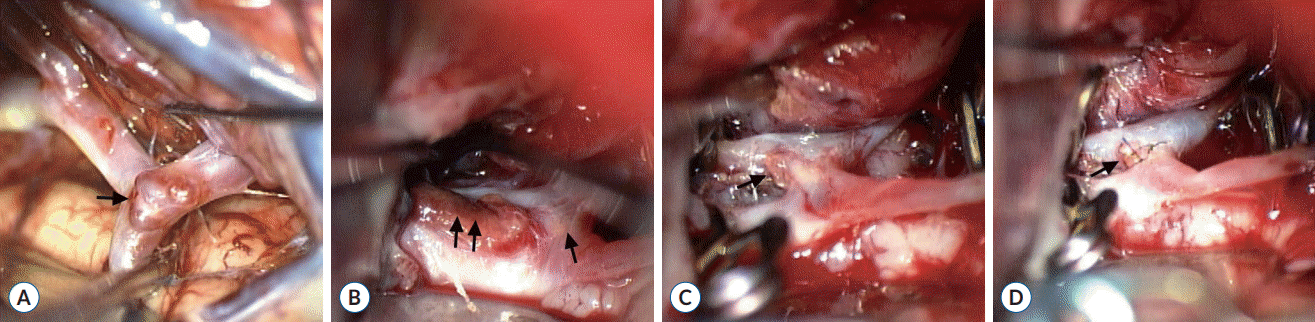
Fig. 3.
Radiological images after the second hemorrhage. A : Computed tomography scan demonstrating an intracerebral hemorrhage (arrow) in the right frontal base starting from the ACoA region and associated intraventricular hemorrhage in the whole ventricular system. B : Digital subtraction angiogram showing no aneurysmal bulge or stenosis in the ACoA (arrow). ACoA : anterior communicating artery.
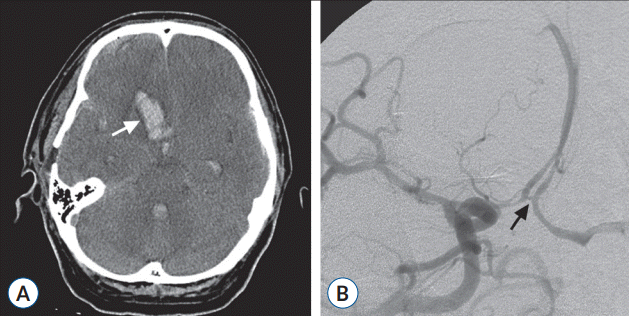
Fig. 4.
Intraoperative photographs of the second operation. A : De novo blister-like aneurysm with a small blood clot (arrow) on the posterosuperior wall of the ACoA close to the right A1/A2 junction. B : Angled fenestrated clip that was placed through the A1 segment with the clip blades occluding the rupture point and ACoA. ACoA : anterior communicating artery.
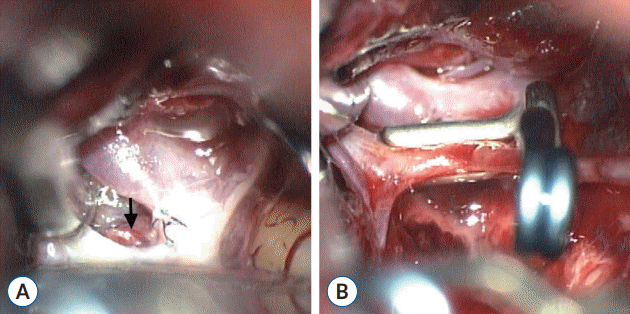
Fig. 5.
Follow-up digital subtraction angiogram 4 years after the initial subarachnoid hemorrhage. A : Right carotid angiogram showing an angled fenestrated clip occluding the rupture point and ACoA on the right side close to the right A1/A2 junction. B : Left carotid angiogram showing an intact ACoA stump (arrow) on the left side, suggesting an intact ACoA perforator. ACoA : anterior communicating artery.
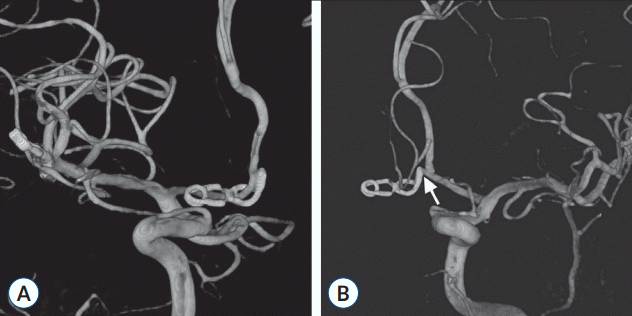
Table 1.
Reported cases with a blister-like ACoA aneurysm
| Study | Age (years)/sex | ACoA aneurysm-related SAH | Initially negative angiogram | Surgically verified diagnosis | Treatment | Treatment-related complication | Aneurysm recurrence | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Andaluz and Zuccarello [3] (2008) | 41/M | + | - | + | Fenestrated clip | - | - | 1 month DSA |
| Andaluz and Zuccarello [3] (2008) | 18/F | + | + | + | Fenestrated clip | - | - | 1 month DSA |
| Andaluz and Zuccarello [3] (2008) | 51/F | + | + | + | Fenestrated clip | - | - | 1 month DSA |
| Andaluz and Zuccarello [3] (2008) | 59/F | + | + | + | Fenestrated clip | - | - | 1 month DSA |
| Andaluz and Zuccarello [3] (2008) | 54/F | + | + | + | Fenestrated clip | - | - | 1 month DSA |
| Morris and Brophy [14] (2009) | 52/M | + | + | + | Fenestrated clip | - | - | 2 months DSA |
| Seo et al. [22] (2009) | 63/M | - | + | + | Clip | - | - | NR |
| Rouchaud et al. [20] (2013) | 61/M | + | - | - | Flow-diverter stent | - | - | 3 months DSA |
| Peschillo et al. [18] (2017) | 70/F | + | + | - | Flow-diverter stent | - | - | 1 week DSA |
| Abiko et al. [2] (2018) | 60/F | + | + | + | Wrapping (temporal fascia) | - | - | 5 months DSA |
| Qian et al. [19] (2019) | 41/M | - | NR | + | Microsutures | - | - | NR |
| Miyashita et al. [13] (2021) | 50/F | + | - | - | Stent | - | - | 3 months DSA |
| Present case | 49/M | + | + | + | Microsutures (left), clip (right) | - | - | 4 years DSA |
| 14 years CTA |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download