To the editor,
The term “paroxysmal sympathetic hyperactivity (PSH)” was first coined by Alejandro Rabinstein in 2007 [4]. The mechanism of PSH has been described as autonomic dysfunction in the diencephalon or its connections that leads to exaggerated sympathetic responses to either internal or external stimuli [2], but the pathophysiology has not been fully explained [6]. The PSH prevalence is much higher in patients with post traumatic brain injury compared with non-traumatic brain injury etiologies [7,8]. Therefore, PSH are often overlooked in relatively rare causes such as brain tumours.
The paroxysms usually begin one week after brain injury, although they may start earlier. The duration of the PSH phase is also variable, ranging from less than 2 weeks to several months [7]. We write this letter to share our experience in managing a case of PSH post brain tumour surgery of glioblastoma multiforme. Recognizing PSH early in the post-operative phase is critical to prevent needless procedures, reduce hospital stay lengths, and improve patient functional outcomes [10].
Our patient is a 36-year-old lady with a history of left parasagittal astrocytoma (World Health Organization [WHO] grade II) post radiotherapy 6 years ago. She presented at the outpatient clinic with seizures and headache for 1 month. Her last brain imaging 4 months before this visit did not show any new lesions. A magnetic resonance imaging of the brain was repeated and new lesions were noted at the left frontal and parietal regions of the brain. She was admitted for debulking of the tumours and the histopathological diagnosis post-surgery was glioblastoma multiforme (WHO grade IIIB).
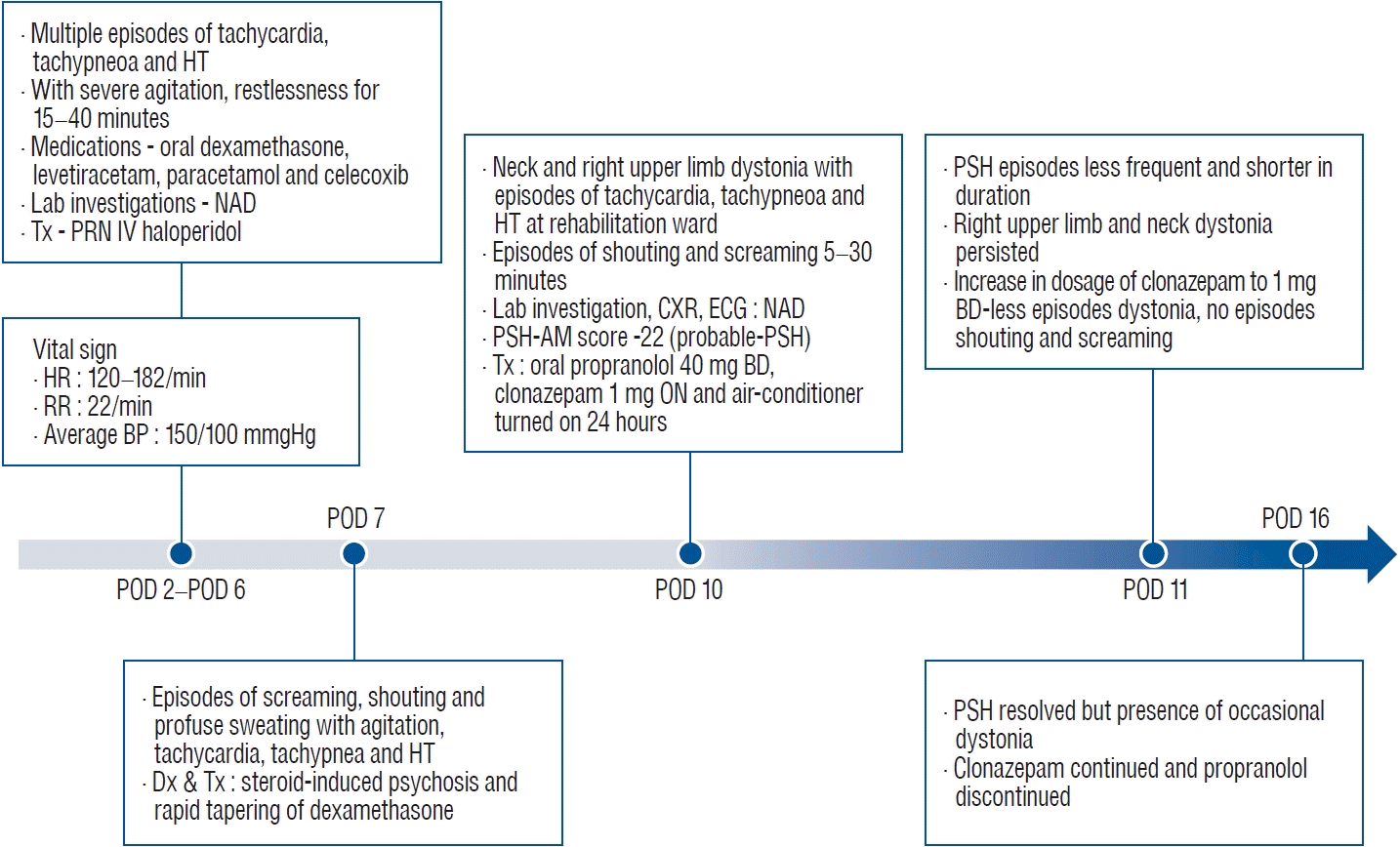
She first developed the symptoms of PSH at day two post brain tumour debulking surgery, with multiple episodes of unprovoked tachycardia, tachypneoa and hypertension. These episodes were accompanied by severe agitation and restlessness which lasted about 15 to 40 minutes. During these episodes, her heart rate ranged from 120 to 182 beats/minute, respiratory rate of 22/minute and an average blood pressure of 150/100 mmHg. These episodes occurred about 4 to 7 times/day. She was stable in between the episodes despite looking lethargic. Her blood results were normal and did not suggest any ongoing sepsis or hypoglycemia.
She was given intravenous haloperidol to treat the episodic agitation and restlessness. Her other medications include oral dexamethasone, levetiracetam, paracetamol and celecoxib. On day 7 post-surgery, she started to have episodes of screaming, shouting and profuse sweating, in addition to the agitation, tachycardia, tachypnea and hypertension observed earlier. She was consolable but only for a short period of time. She refused to eat, communicate, or participate in any bedside assessment or therapy. She was then diagnosed as having a possible dexamethasone-induced psychosis and tapering down dexamethasone was hastened.
Differential diagnoses such as sepsis, hypoxia and other life-threatening conditions were ruled out. The diagnosis of PSH was not made during the first 9 days of presentation, and she was only treated with intravenous haloperidol and regular paracetamol. The symptoms, however, did not resolve. Rehabilitation was also delayed due to the patient’s refusal to cooperate, and the disturbances from the episodic symptoms.
PSH was first suspected when the patient was transferred to the rehabilitation ward. The presentation of sudden episodic manifestations of sympathetic activities that disappeared completely in between episodes raised a high suspicion index. The patient also remained well in between episodes. This is an important feature that differentiates PSH from life threatening emergencies that have similar presentation. Since PSH is a clinical diagnosis, the PSH-assessment measure proposed by Baguley [1] was used to aid the clinical diagnosis and monitor severity of symptoms over time.
Treatment for PSH was initiated according to existing recommendations which consists of general care measures and pharmacologic therapy with abortive medications [3,7]. The combination of benzodiazepine (clonazepam), and beta-adrenergic blocker (propranolol) was effective for our patient. The air conditioner and standing fan were added as measures to reduce sweating [9]. The frequency and intensity of the symptoms became less within 24 hours of starting treatment and completely resolved after 5 days except for dystonia. Fig. 1 outlines the timeline of the event, beginning from the onset of PSH symptoms to its resolution.
It is possible for patients with PSH to have residual dystonia and spasticity [2] as in our patient’s case. If left untreated, secondary complications may occur which include weight loss and muscle contractures [5]. We were unable to monitor the complete resolution of dystonia as the patient did not return for her appointment. However, the dystonia was much less in frequency and severity which has allowed her to participate in therapy and functional training before discharged.
In conclusion, there should be a high suspicion of PSH in patients with brain tumour during the acute period of post-tumour debulking surgery. Early diagnosis of PSH allows the necessary management to be initiated and prevents delay in improving a patient’s function. It can also prevent unnecessary testing and prolonged duration of hospital stay. The prompt diagnosis and treatment are crucial in improving functional outcomes in patients with PSH.
Notes
References
1. Baguley IJ. The excitatory:inhibitory ratio model (EIR model): an integrative explanation of acute autonomic overactivity syndromes. Med Hypotheses. 70:26–35. 2008.

2. Baguley IJ, Perkes IE, Fernandez-Ortega JF, Rabinstein AA, Dolce G, Hendricks HT, et al. Paroxysmal sympathetic hyperactivity after acquired brain injury: consensus on conceptual definition, nomenclature, and diagnostic criteria. J Neurotrauma. 31:1515–1520. 2014.

3. Lee S, Jun GW, Jeon SB, Kim CJ, Kim JH. Paroxysmal sympathetic hyperactivity in brainstem-compressing huge benign tumors: clinical experiences and literature review. Springerplus. 5:340. 2016.

4. Meyfroidt G, Baguley IJ, Menon DK. Paroxysmal sympathetic hyperactivity: the storm after acute brain injury. Lancet Neurol. 16:721–729. 2017.

5. Rabinstein AA : Paroxysmal sympathetic hyperactivity. Available at : https://www.uptodate.com/contents/paroxysmal-sympathetichyperactivity.
6. Rabinstein AA. Paroxysmal sympathetic hyperactivity in the neurological intensive care unit. Neurol Res. 29:680–682. 2007.

7. Rabinstein AA, Benarroch EE. Treatment of paroxysmal sympathetic hyperactivity. Curr Treat Options Neurol. 10:151–157. 2008.

8. Suliman MS, Dobariya V, Shehata M, Singh D, Al-Astal A. Paroxysmal sympathetic hyperactivity in a young male with glioblastoma multiforme. Cureus. 12:e6933. 2020.

Fig. 1.
Timeline of clinical events and treatment. HT : hypertension, NAD : no abnormalities detected, Tx : treatment, PRN : pro re nata, IV : intravenous, HR : heart rate, RR : respiratory rate, BP : blood pressure, CXR : chest X-ray, ECG : electrocardiogram, PSH : paroxysmal sympathetic hyperactivity, AM : assessment measure, BD : bis in die, ON : omni nocte, POD : postoperative day.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download