Abstract
Objective
Microscopic microvascular decompression (MVD) has been considered to be a useful treatment modality for medically refractory hemifacial spasm (HFS) and trigeminal neuralgia. But, the advent of the endoscopic era has presented new possibilities to MVD surgery. While the microscope remains a valuable tool, the endoscope offers several advantages with comparable clinical outcomes. Thus, fully endoscopic MVD (E-MVD) could be a reasonable alternative to microscopic MVD. This paper explores the safety and efficacy of the fully E-MVD technique.
Methods
A single-center retrospective study was conducted in 25 patients diagnosed with HFS between September 2019 and July 2023. All surgeries were performed by a single neurosurgeon using the fully E-MVD technique without any assistance of a microscope. The study reviewed intraoperative brainstem auditory evoked potentials and disappearance of the lateral spread response. Outcomes were assessed based on the patients’ clinical status immediately after surgery and at their last follow-up. Complications, including facial palsy, hearing loss, ataxia, dysphagia, palsy of other cranial nerves, and cerebrospinal fluid leakage, were also examined.
Results
The most common offending artery was the anterior inferior cerebellar artery (AICA) in 15 cases (60.0%), followed by the posterior inferior cerebellar artery in eight cases (32.0%), vertebral artery (VA) in one case (4.0%), tandem lesions involving the AICA and VA in one case (4.0%). Ten patients (40.0%) had pre-operative facial palsy on the ipsilateral side, and eight patients (32.0%) experienced delayed facial palsy on the ipsilateral side, from which they fully recovered by the last follow-up. The median operation time was 105 minutes. All patients were symptom free immediately after surgery and at the last follow-up. One patient experienced a permanent complication, such as high-frequency hearing loss, from which he partially recovered over time.
Conclusion
Fully E-MVD demonstrated similar clinical outcomes to microscopic MVD. It offered a similar complication rate, shorter operation time, and a panoramic view with a smaller craniectomy size. Although there is a learning curve associated with fully E-MVD, it presents a viable alternative in the endoscopic era.
The distinct symptom of hemifacial spasm (HFS) is involuntary movement of facial muscles innervated by the facial nerve [9]. It is generally accepted that HFS is caused by facial nerve root exit zone (REZ) myelin breakdown and ephaptic transmission [9]. The REZ of the facial nerve is the transition point between central and peripheral cell myelination [9,10]. This segment of the facial nerve is only protected by the arachnoid mater and thus easily damaged by vascular compression [10]. The most commonly involved arteries are the posterior inferior cerebellar artery (PICA) and anterior inferior cerebellar artery (AICA) [12].
Microvascular decompression (MVD) for HFS has been used as a useful and effective treatment modality [1,2,9,14]. Adequate separation of the involved arteries from the REZ of the nerve is important, and Teflon is the most commonly used separating material. Microscopic MVD is still a good tool for MVD surgery, but the endoscope offers several advantages with similar clinical outcomes [3-6,8,11,15]. The panoramic view afforded by the endoscope enlarges the surgical field of vision while eliminating cerebellar or brainstem retraction and the need for extensive dissection, which is necessary when using the microscope, to allow for an unobstructed view of the relevant neurovascular structures [5]. The current study aimed to describe the safety and efficacy of the fully endoscopic MVD (E-MVD) technique without any assistance of the microscope. We thought that the fully E-MVD technique is not difficult for a beginner who is not familiar with endoscopy in the perspective of a learning curve.
This study was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (SCHCA 2023-12-035).
A single-center retrospective study was conducted in 25 patients who were enrolled consecutively. All patients were evaluated in terms of the pre- and post-operative clinical status. We collected medical data related to demographics, preoperative symptoms, the offending vessel, operation time, surgical outcomes, and postoperative complications. Preoperative electrophysiological examinations were conducted to confirm the presence of abnormalities in the blink reflex and facial nerve conduction. Additionally, prior to surgery, magnetic resonance imaging (MRI) was performed using the volume isotropic turbo spin echo acquisition (VISTA) sequence with a focus on the brain stem to preoperatively identify the offending vessel.
All operations were performed by a single neurosurgeon with the fully E-MVD technique without any assistance of a microscope. Surgeries were performed under general anesthesia, with the patient in a supine position and ipsilateral head rotation at 90 degrees. A 3-pin head fixator was used for head stabilization. For surgery, a 4 mm 0-degree and 30-degree rigid endoscope were utilized (IMAGE1 STM RUBINATM; Karl Storz SE & Co. KG, Tuttlingen, Germany), and a manually operated endoscopic fixation device was used to secure the endoscope to the bed.
The surgical procedure was performed in a similar manner to that of microscopic MVD, but with some modifications. Instead of the typical retromastoid approach, a shorter linear skin incision was made, followed by a burr hole craniectomy to expose the sigmoid sinus and transverse sinus (Fig. 1). More than one third of the sigmoid sinus was exposed to ensure a clear view and angle for entry of the endoscope. A C-shaped dural incision was made along the boundary of the sinus, and cerebrospinal fluid (CSF) was drained with slow rate to gently separate the cerebellum from the dura.
For effective CSF drainage, several conditions were necessary. Firstly, the patient’s position must be adjusted to a classic 15-degree vertex down. Secondly, the craniectomy must be located above the low cranial nerves (CNs), making adjustments to allow a vertical view of the cerebellomedullary cistern by drilling the skull’s inner table. If CSF drainage was not conducted in a timely manner, it might be necessary to retract the cerebellum to open the cistern and secure an adequate view, which could be challenging. After ensuring enough CSF drainage and relaxing the cerebellum, the endoscope was carefully inserted. Once the endoscope was mounted on the endoscope holder, the arachnoid membrane was dissected to carefully expose and identify the CNs, especially the REZ of the facial nerve. It was crucial to completely dissect the arachnoid membrane around the low CNs to allow for a tension-free release of the low CNs and to prevent unnecessary damage. When instruments such as suction were introduced alongside the endoscope, they should enter ahead of the endoscope. Especially before becoming accustomed to the endoscope, introducing instruments after the endoscope increased the risk of causing damage to the cerebellum. Using a cottonoid patty at the access corridor (lateral to the cerebellar cortex), the instruments were safely inserted from the dura mater to the operative field, inspecting them with a macroscopic view. The 30-degree endoscope provided a more accurate anatomical position and panoramic view compared to the 0-degree endoscope, but caution was exercised due to spatial distortion. When entering with the endoscope, it would be safe to approach parallel to the petrous bone. Additionally, creating an angle that feels like looking down from above would be safe rather than directly facing the objectives.
When the offending vessel was identified, Teflon (TFE Polymer Pledget; Ethicon, Inc., Raritan, NJ, USA) was inserted using forceps and secured with fibrin glue. Microscopic MVD involves performing surgery while looking from the front. But, E-MVD is performed by looking down from above, and it facilitated that the extent of retraction is naturally decreased with direct visualization of the REZ. Moreover, using suction and curved instruments during endoscopic surgery is thought to offer slight improvements compared to microscopic surgery. The dura was sutured tightly to ensure it was watertight, and a burr hole-type miniplate was used to cover the bony defect. The operating time was defined as from the start of skin incision to the end of skin closure.
IONM included triggered CN electromyography, brainstem auditory evoked potentials (BAEPs), and disappearance of the lateral spread response (LSR). IONM was performed by using the NIM-Eclipse (Medtronic, Minneapolis, MN, USA) system.
We assessed the outcomes by evaluating patients’ clinical status immediately after surgery and at their last follow-up. We conducted a separate analysis for acute complications occurring before discharge and delayed complications observed during the outpatient follow-up period. We described the types of complications, such as facial paralysis and hearing loss, individually. Additionally, we examined the findings from the last follow-up examination.
Patient demographics are summarized in Table 1. There were nine men (36.0%) and 16 women (64.0%). The median age was 55 years (range, 35–72). Eleven cases showed right-sided disease (44.0%) and 14 cases showed left-sided disease (56.0%). Among these 25 patients, 10 patients (40.0%) had pre-operative facial palsy on the ipsilateral side, which was graded as House-Brackmann grade 2. The asymmetry of mouth corner, deep nasolabial fold caused by long-standing spasm of affected muscles was included as facial palsy.
The most common offending artery was the AICA in 15 cases (60.0%), followed by the PICA in eight cases (32.0%), the vertebral artery (VA) in one case (4.0%), and a tandem offender involving the AICA and VA in one case (4.0%).
Disappearance of the LSR was confirmed in all patients (100.0%). The median operating time was 105 minutes with a range from 65 to 230 minutes.
All patients were symptom free at the immediate post-operative neurologic examination. The follow-up period ranged from 3 to 49 months, with a median follow-up duration of 24 months. All patients remained symptom free at the last follow-up examination. However, in two patients (7.4%), there was a recurrence of symptoms 2 months after surgery. These individuals were successfully managed with carbamazepine, and at their most recent follow-up, they achieved stable symptom control without the need for medication.
In one patient, post-operative hearing impairment was observed, and high-frequency hearing loss was confirmed on audiometric testing. Another patient experienced CSF leakage after surgery, which was managed with a lumbar drain for 5 days, and the patient was discharged without any further complications. Delayed facial palsy occurred in a total of eight patients (32.0%) approximately 2 months after surgery. However, all these patients showed improvement following oral corticosteroid treatment, and at their last follow-up, they exhibited normal facial function. No other complications, such as intracranial hemorrhage, cerebral infarction, or CN injuries, were observed.
A 64-year-old man was admitted with a chief complaint of right-sided HFS. He had been experiencing HFS for 3 years, with worsening of symptoms over the past 1 month. Despite receiving oral medications and BOTOX® (clostridium botulinum toxin type A; AbbVie, Inc., North Chicago, IL, USA) treatment, his symptoms did not improve. Consequently, we decided to proceed with surgical MVD due to the refractory nature of his condition to medical treatment. MRI with the VISTA sequence revealed that the right AICA and right VA were compressing the right facial nerve at the REZ (Fig. 2A). Dolichoectasia of the VA was also observed, and the VA diameter was approximately 8 mm. Preoperative electromyogram motor nerve conduction velocity (EMG-NCV) testing of the facial nerve was conducted to establish baseline data for comparison between before and during the operation. Prior to surgery, baseline data indicated the presence of HFS. Pure tone audiometry and speech audiometry were performed to assess hearing impairments, but no specific findings were noted.
After standard linear skin incision and burr hole craniectomy, we opened the cerebellomedullary cistern and cerebellar relaxation was achieved with CSF drainage. Following arachnoid dissection, we identified the CN VII & VIII complex and lower CNs. Simultaneously, we observed that the dolichoectatic right VA and right AICA were compressing CN 7 at the REZ (Fig. 2B). We attempted gentle mobilization of the VA for better visualization, and then interposed Teflon (TFE Polymer Pledget; Ethicon, Inc.) between the nerve and vessel. A total of four pieces of Teflon were embedded (Fig. 2C), resulting in the disappearance of LSR. However, the LSR recurred when the retracted VA returned to its original position. Therefore, we prepared for transposition of the VA. The VA diameter was measured, and a sling (Bard® PTFE Felt Pledget; Bard Peripheral Vascular, Inc., Tempe, AZ, USA) was designed with dimensions of approximately 4 mm in width and 30 mm in length. The VA was encircled by the sling without interference by any small vessels during the passage. We then mobilized the sling towards the posterior petrous bone and sutured it to the dura of the petrous bone, transposing the VA away from its original position (Fig. 2D). After one suture, the transposed VA was abutting the lower CNs, prompting the creation of another suture in a more superolateral direction (Fig. 2E). This resulted in the release of compression on the lower CNs, and there was no obstruction at the REZ of CN 7 anymore. Ultimately, the LSR permanently disappeared after the transposition of the dolichoectatic VA using the sling technique. Throughout the entire operation, BAEP monitoring was conducted, and no significant findings were observed (Supplementary Video 1).
To our knowledge, microscopic MVD has been the standard treatment modality for HFS and TN. In 1995, Barker et al. [2] reported that MVD was the treatment of choice for most of the HFS cases, with low rates of delayed recurrence and operative morbidity. The following year, Barker et al. [1] reported the long-term outcome of MVD for TN in 1185 patients, emphasizing the safety and efficacy of MVD with a high rate of long-term success. In 2002, Jarrahy et al. [7] presented the first case report of fully E-MVD for TN. Recently, in 2018, Flanders et al. [5] reported 28 cases of fully E-MVD for HFS and concluded that it was a safe and effective approach. In 2018, Zagzoog et al. [15] reported a systematic review and comparative meta-analysis of E-MVD versus open MVD for TN. They found that the clinical outcomes with respect to pain relief were similar between MVD and E-MVD. The recurrence rate was lower with E-MVD, and the complication rates were statistically higher with microscopic MVD than with E-MVD. They concluded that the E-MVD technique offered good surgical outcomes with several advantages, including shorter operative time, smaller craniotomy, and lower recurrence rates.
There are several advantages of fully E-MVD. First of all, the endoscope provides the panoramic view of the cerebellopontine angle and it can enlarge the surgical field without any retraction. In microscopic MVD, cerebellar or brainstem retraction is often necessary, which can lead to retraction-related damage to CNs or the cerebellum. E-MVD allows closer inspection of the nerve and its surrounding structures at difficult angles, with excellent visualization of the entire length of the nerve [11]. Additionally, the panoramic view reduces the need to track the offending vessel, which may not always be inspected with a microscope. In general, the fully E-MVD technique requires a more smaller scalp incision and burr hole craniectomy compared to microscopic MVD; thus, reducing the operative time, hospitalization period, and costs.
However, fully E-MVD can be challenging for achieving bleeding control. Without any additional assistance, the surgeon should hold the endoscope and it would be difficult to control the bleeding compared to the microscope. Moreover, if the surgeon is not familiar with the endoscope, a long-term learning curve may be needed. Phang et al. [13] assessed the learning curve of MVD performed by a beginner. They suggested that beginners can become accustomed to MVD after performing their first eight cases within 40 days. The learning curve for fully E-MVD may not be significantly longer than for microscopic MVD. Despite disadvantages, such as bleeding control and learning curve, fully E-MVD is beneficial for both the surgeons and patients. Therefore, we consider that fully E-MVD is a good alternative to microscopic MVD.
This study is limited due to the small sample size, lack of a control group, and short-term follow-up duration. Comparison with a control group and long-term clinical follow-up will help to elucidate the advantages of fully E-MVD.
The fully E-MVD is a promising alternative to traditional microscopic MVD for the treatment of medically refractory HFS. With minimal complication rate, the study highlighted the advantages of fully E-MVD, including a shorter operation time, smaller craniectomy size, and a panoramic view without the need for extensive tissue retraction.
Despite potential challenges, such as bleeding control and a learning curve associated with the endoscopic technique, fully E-MVD appears to be a viable and safe option for neurosurgeons. Further studies with a larger sample size, control group, and long-term follow-up are warranted to validate the advantages of fully E-MVD over microscopic MVD. As the endoscopic era progresses, fully E-MVD stands as a valuable addition to the surgical armamentarium for MVD surgery, offering potential benefits for both surgeons and patients alike.
Notes
Informed consent
Informed consent was obtained from all individual participants included in this study.
ACKNOWLEDGMENTS
This work was supported by the Soonchunhyang University Hospital Cheonan Research Fund.
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2024.0003.
References
1. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 334:1077–1084. 1996.

2. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 82:201–210. 1995.

3. Bohman LE, Pierce J, Stephen JH, Sandhu S, Lee JYK. Fully endoscopic microvascular decompression for trigeminal neuralgia: technique review and early outcomes. Neurosurg Focus. 37:E18. 2014.

4. Cheng WY, Chao SC, Shen CC. Endoscopic microvascular decompression of the hemifacial spasm. Surg Neurol 70 Suppl. 1:40–46. 2008.

5. Flanders TM, Blue R, Roberts S, McShane BJ, Wilent B, Tambi V, et al. Fully endoscopic microvascular decompression for hemifacial spasm. J Neurosurg. 131:813–819. 2018.

6. Halpern CH, Lang SS, Lee JYK. Fully endoscopic microvascular decompression: our early experience. Minim Invasive Surg. 2013:739432. 2013.

7. Jarrahy R, Eby JB, Cha ST, Shahinian HK. Fully endoscopic vascular decompression of the trigeminal nerve. Minim Invasive Neurosurg. 45:32–35. 2002.

8. Lee JYK, Pierce JT, Sandhu SK, Petrov D, Yang AI. Endoscopic versus microscopic microvascular decompression for trigeminal neuralgia: equivalent pain outcomes with possibly decreased postoperative headache after endoscopic surgery. J Neurosurg. 126:1676–1684. 2017.

9. Lu AY, Yeung JT, Gerrard JL, Michaelides EM, Sekula RF Jr, Bulsara KR. Hemifacial spasm and neurovascular compression. ScientificWorldJournal. 2014:3493. 2014.

10. Nielsen VK. Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology. 34:418–426. 1984.
11. Pak HL, Lambru G, Okasha M, Maratos E, Thomas N, Shapey J, et al. Fully endoscopic microvascular decompression for trigeminal neuralgia: technical note describing a single-center experience. World Neurosurg. 166:159–167. 2022.

12. Park JS, Kong DS, Lee JA, Park K. Hemifacial spasm: neurovascular compressive patterns and surgical significance. Acta Neurochir (Wien). 150:235–241. discussion 241. 2008.

13. Phang SY, Martin J, Zilani G. Assessing the safety and learning curve of a neurosurgical trainee in performing a microvascular decompression (MVD). Br J Neurosurg. 33:486–489. 2019.

Fig. 1.
The typical approach method for fully endoscopic microvascular decompression. A : Following a linear skin incision, we performed a burr hole craniectomy. The size of the burr hole did not exceed the dimensions suitable for a large-sized burr hole miniplate. B : We conducted a burr hole craniectomy to expose the sigmoid sinus (white arrow) and transverse sinus (yellow arrow). This image serves as an illustrative example of trigeminal neuralgia in our study.
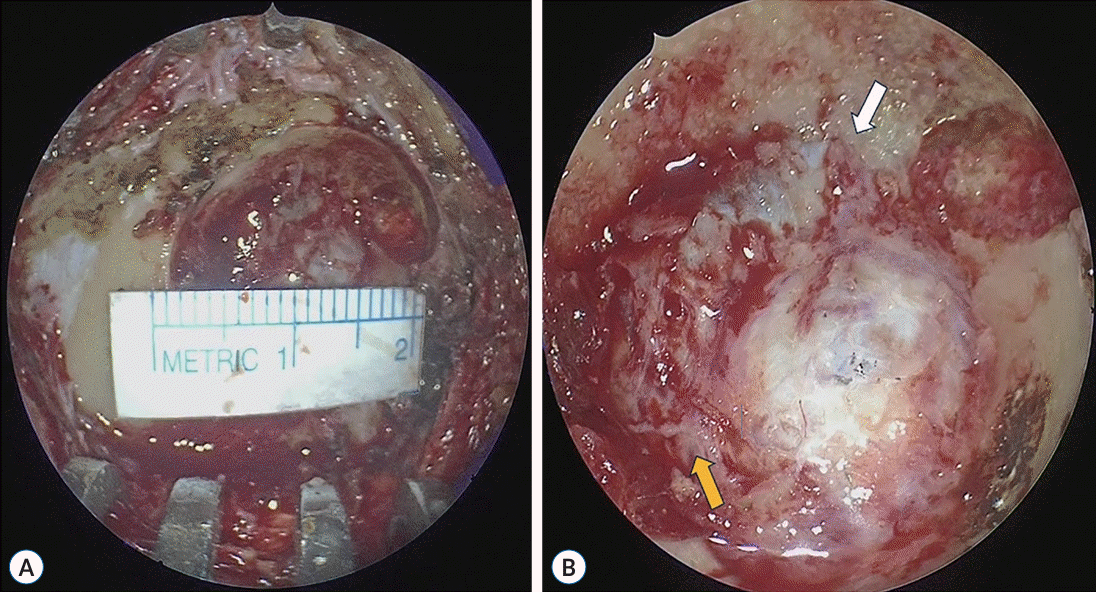
Fig. 2.
Case presentation, intraoperative view of fully endoscopic microvascular decompression. A : Magnetic resonance imaging (MRI) revealed the right anterior inferior cerebellar artery and right dolichoectatic vertebral artery (VA) abutting the right facial nerve at the root exit zone (REZ) (white arrow). B : In the endoscopic view, we could confirm the MRI finding of dolichoectatic VA (yellow arrow) compressing the cranial nerve (CN) 7 (blue arrow) at the REZ. C : After interposition of Teflon, the lateral spread response disappeared for a while. D : Transposition of the offending vessel with the sling technique. Another suture was needed for minimizing compression of the lower CN. E : Final view after decompression and sling.
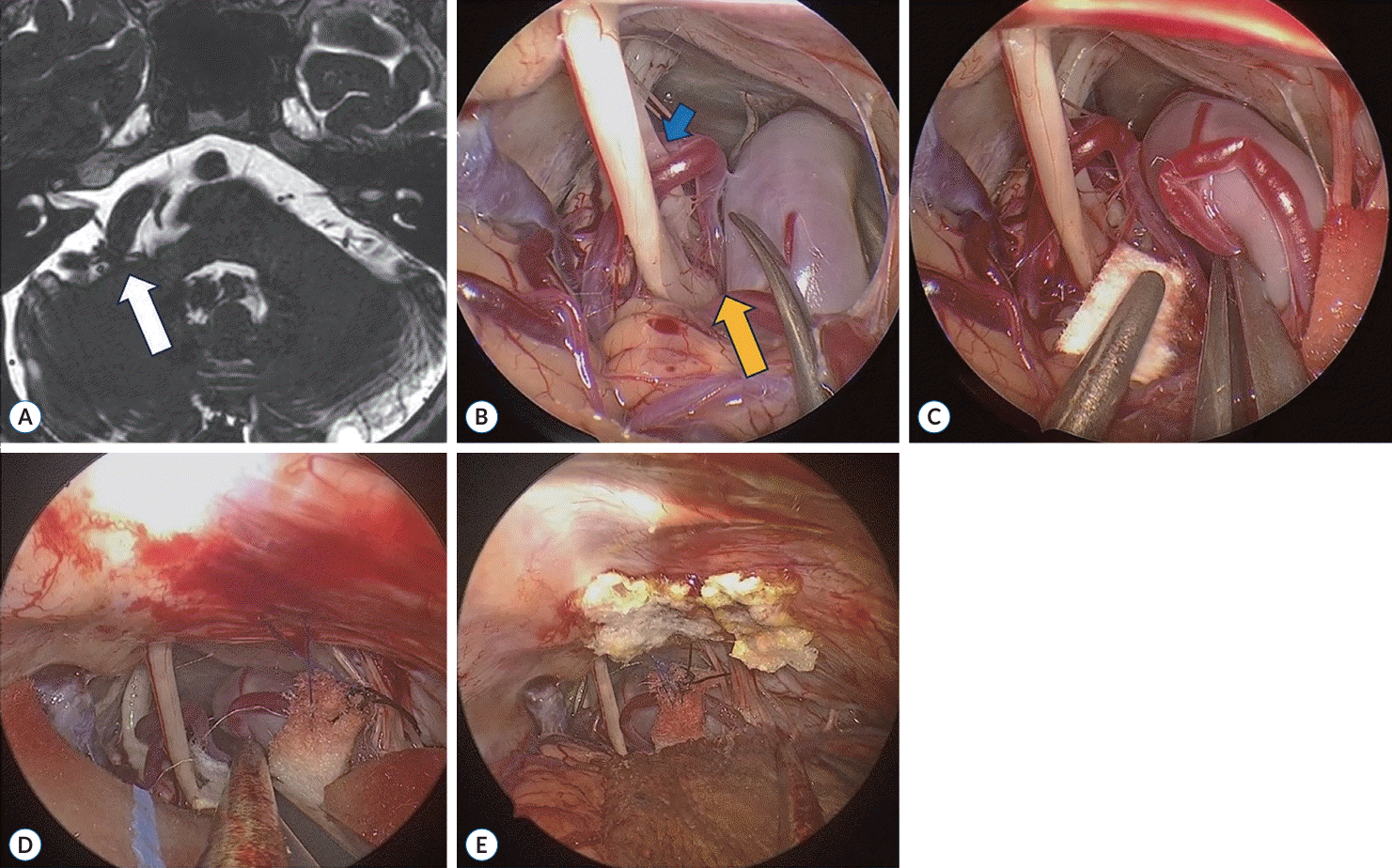
Table 1.
Patient demographics, intraoperative findings, and postoperative outcomes




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download