Abstract
Objective
Methods
Results
Notes
Author contributions
Conceptualization : BCL, YWL, GOK, JSO; Data curation : SWP, JJH, NHH, JSO; Formal analysis : SWP, JJH, NHH, JSO; Funding acquisition : JSO; Methodology : SWP, JJH, NHH, JSO; Project administration : BCL, YWL, GOK, JSO; Visualization : ECL, DHL, JYL, JSO; Writing - original draft : SWP, JJH, NHH, JSO; Writing - review & editing : SWP, JJH, JSO
ACKNOWLEDGMENTS
Supplementary materials
Supplementary Table 1.
Supplementary Table 2.
References
Fig. 1.
Fig. 2.
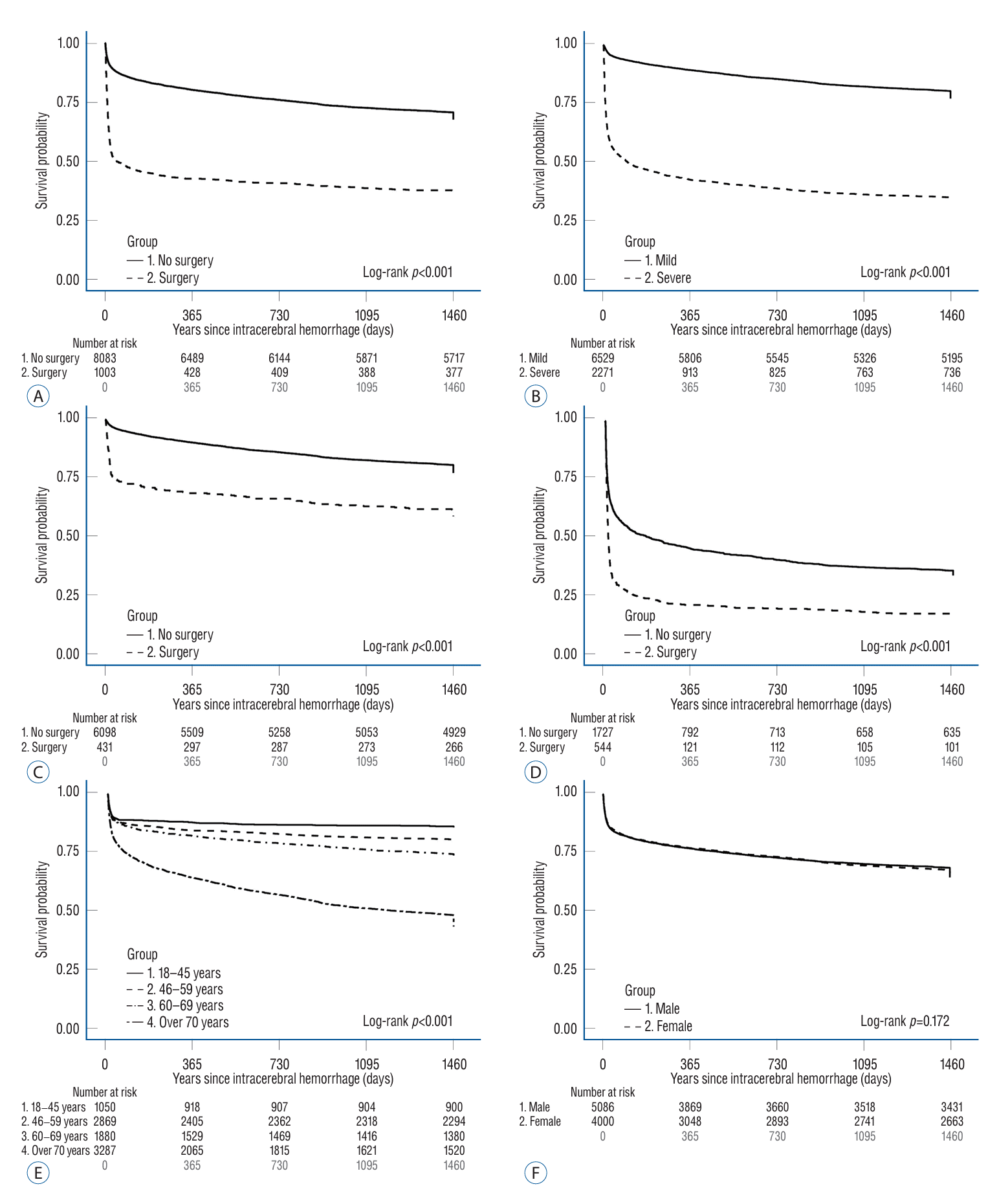
Fig. 3.
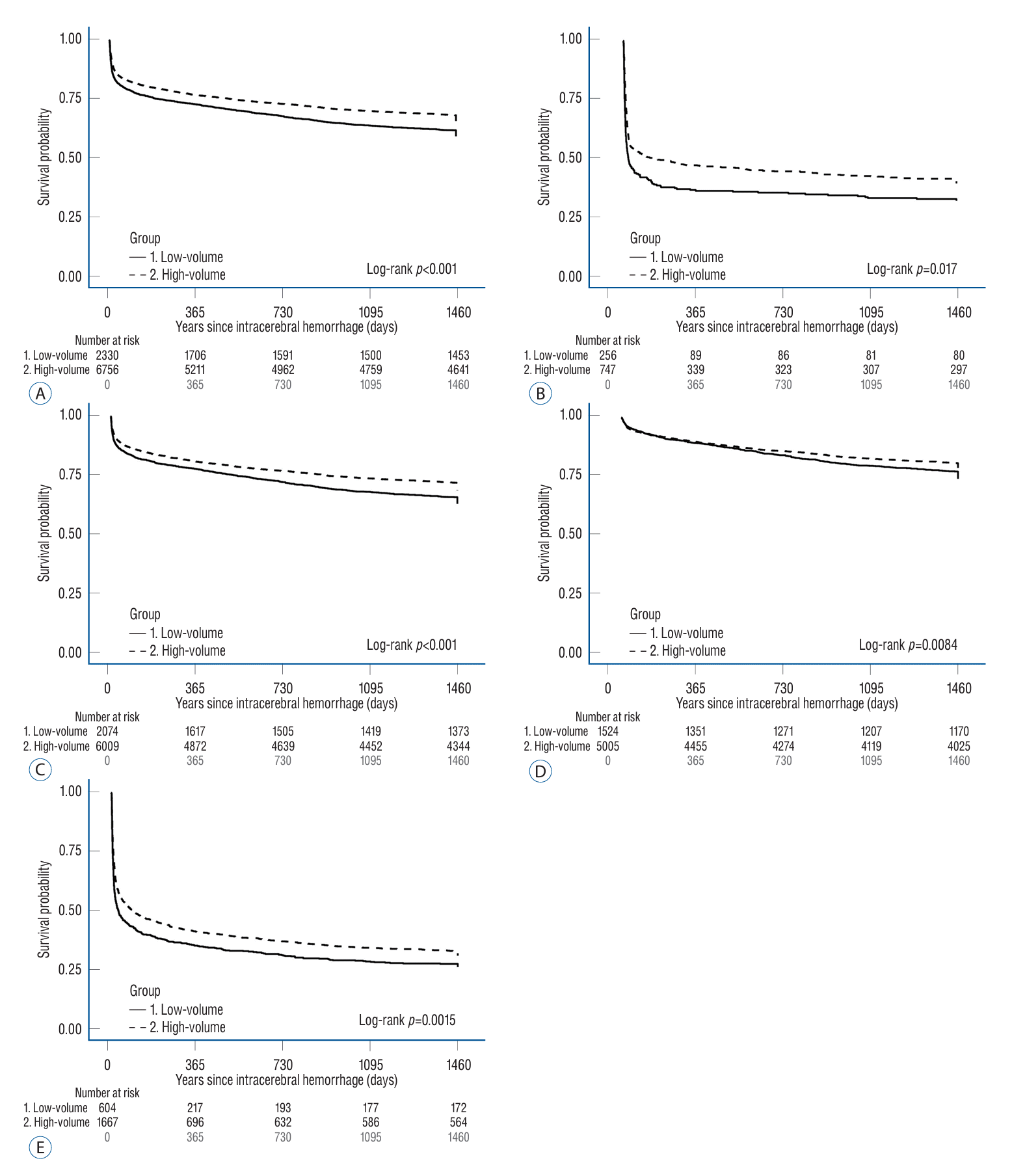
Table 1.
| Variable | Total | Low volume hospital | High volume hospital | p-value |
|---|---|---|---|---|
| Total number of patients | 9086 (100.00) | 2330 (25.64) | 6756 (74.36) | |
| Total number of hospitals | 263 (100.00) | 185 (70.34) | 78 (29.66) | |
| Age | ||||
| 18–45 years | 1050 (11.55) | 240 (10.30) | 810 (11.99) | <0.001* |
| 46–59 years | 2869 (31.58) | 672 (28.84) | 2197 (32.52) | |
| 60–69 years | 1880 (20.69) | 474 (20.34) | 1406 (20.81) | |
| ≥70 years | 3287 (36.18) | 944 (40.52) | 2343 (34.68) | |
| Gender | ||||
| Male | 5086 (55.98) | 1268 (54.42) | 3818 (56.51) | 0.079 |
| Female | 4000 (44.02) | 1062 (45.58) | 2938 (43.49) | |
| Health insurance type | ||||
| Health insurance | 4558 (92.27) | 1216 (90.68) | 3342 (92.86) | 0.011* |
| Medical aid | 382 (7.73) | 125 (9.32) | 257 (7.14) | |
| GCS score | ||||
| 13–15 | 5528 (62.82) | 1287 (60.48) | 4241 (63.56) | 0.008 |
| 9–12 | 1404 (15.95) | 340 (15.98) | 1064 (15.95) | |
| 0–8 | 1868 (21.23) | 501 (23.54) | 1367 (20.49) | |
| Severity | ||||
| Mild | 6529 (74.19) | 1524 (71.62) | 5005 (75.01) | 0.002* |
| Severe | 2271 (25.81) | 604 (28.38) | 1667 (24.99) | |
| Medical history | ||||
| Smoker | ||||
| Current smoker | 1052 (19.59) | 247 (19.30) | 805 (19.68) | 0.395 |
| Ex-smoker | 418 (7.78) | 111 (8.67) | 307 (7.51) | |
| Non-smoker | 3900 (72.63) | 922 (72.03) | 2978 (72.81) | |
| CCI score | ||||
| 0 | 1737 (19.12) | 416 (17.85) | 1321 (19.55) | 0.187 |
| 1 | 1413 (15.55) | 370 (15.88) | 1043 (15.44) | |
| 2 | 1548 (17.04) | 421 (18.07) | 1127 (16.68) | |
| ≥3 | 4388 (48.29) | 1123 (48.20) | 3265 (48.33) | |
| Arrival mode | ||||
| EMS | 6747 (74.36) | 1680 (72.51) | 5067 (75.00) | 0.018* |
| No EMS | 2326 (25.64) | 637 (27.49) | 1689 (25.00) | |
| Onset to door time | ||||
| ≤4.5 hours | 6431 (70.78) | 1654 (70.99) | 4777 (70.71) | 0.798 |
| >4.5 hours | 2655 (29.22) | 676 (29.01) | 1979 (29.29) | |
| Facility type | ||||
| Tertiary hospital | 4090 (45.01) | 143 (6.14) | 3947 (58.42) | <0.001* |
| General hospital | 4996 (54.99) | 2187 (93.86) | 2809 (41.58) | |
| Door to image time | ||||
| ≤1 hour | 7216 (93.22) | 1814 (93.65) | 5402 (93.07) | 0.383 |
| >1 hour | 525 (6.78) | 123 (6.35) | 402 (6.93) | |
| Stroke unit | ||||
| Yes | 5322 (58.57) | 664 (28.50) | 4658 (68.95) | <0.001 |
| No | 3764 (41.43) | 1666 (71.50) | 2098 (31.05) | |
| Surgery type | ||||
| No surgery | 8083 (88.96) | 2074 (89.01) | 6009 (88.94) | 0.926 |
| Surgery | 1003 (11.04) | 256 (10.99) | 747 (11.06) | |
| Functional outcome at discharge | ||||
| Good outcome | 3379 (37.83) | 1538 (68.29) | 4014 (60.10) | <0.001 |
| Poor outcome | 5552 (62.17) | 714 (31.71) | 2665 (39.90) | |
| mRS (52.60%) | ||||
| 0 | 389 (8.14) | 78 (7.25) | 311 (8.40) | 0.012* |
| 1 | 1153 (24.13) | 257 (23.88) | 896 (24.20) | |
| 2 | 755 (15.80) | 158 (14.68) | 597 (16.12) | |
| 3 | 579 (12.12) | 126 (11.71) | 453 (12.23) | |
| 4 | 966 (20.21) | 211 (19.62) | 755 (20.39) | |
| 5 | 620 (12.97) | 148 (13.75) | 472 (12.75) | |
| 6 | 317 (6.63) | 98 (9.11) | 219 (5.91) | |
| GOS (20.04%) | ||||
| 1 | 179 (9.83) | 32 (10.16) | 147 (9.76) | 0.002* |
| 2 | 123 (6.75) | 28 (8.89) | 95 (6.31) | |
| 3 | 356 (19.55) | 78 (24.76) | 278 (18.46) | |
| 4 | 477 (26.19) | 87 (27.62) | 390 (25.90) | |
| 5 | 686 (37.68) | 90 (28.57) | 596 (39.57) | |
| Number of deaths | ||||
| 3-month (2013, 2014, 2016, 2018) | 1658 (18.25) | 490 (21.03) | 1168 (17.29) | <0.001* |
| 1-year (2013, 2014, 2016, 2018) | 2169 (23.87) | 624 (26.78) | 1545 (22.87) | <0.001* |
| 2-year (2013, 2014, 2016, 2018) | 2533 (27.88) | 739 (31.72) | 1794 (26.55) | <0.001* |
| 4-year (2013, 2014, 2016; n=5607) | 2004 (35.74) | 600 (41.64) | 1404 (33.70) | <0.001* |
Values are presented as number (%). Mild severity = NIHSS ≤15, GCS ≥10; severe severity = NIHSS ≥16, GCS ≤9. Good outcome : K-MBI (75–99), MBI (75–99), BI (75–99), mRS (0–2), FIM (90–126), GOS (5). Patients who had no record of functional outcome at discharge were excluded. mRS was used in 52.6%, and GOS was 20.4%.
GCS : Glasgow coma scale, CCI : Charlson comorbidity index, EMS : emergency medical services, mRS : modified Rankin scale, GOS : Glasgow outcome scale, NIHSS : National Institutes of Health Stroke Scale, K-MBI : Korean version of modified Barthel index, MBI : modified Barthel index, BI : Barthel index, FIM : Functional independence measure
Table 2.
|
Poor outcome at discharge |
||
|---|---|---|
| OR (95% CI) | p-value | |
| Medical facility type | ||
| Low-volume hospital | 1.0 | |
| High-volume hospital | 0.81 (0.72–0.91)* | <0.001 |
| Surgery type | ||
| No surgery | 1.0 | |
| Surgery | 2.31 (1.90–2.81)* | <0.001 |
| Severity | ||
| Mild | 1.0 | |
| Severe | 8.50 (7.20–10.02)* | <0.001 |
| Age | ||
| 18–45 years | 1.0 | |
| 46–59 years | 1.08 (0.91–1.28) | 0.386 |
| 60–69 years | 1.28 (1.07–1.53)* | 0.007 |
| ≥70 years | 2.24 (1.89–2.66)* | <0.001 |
| Gender | ||
| Male | 1.0 | |
| Female | 1.01 (0.91–1.12) | 0.893 |
| Arrival mode | ||
| EMS | 1.0 | |
| No EMS | 0.40 (0.36–0.45)* | <0.001 |
| Medical history | ||
| CCI score | ||
| 0 | 1.0 | |
| 1 | 1.09 (0.92–1.29) | 0.325 |
| 2 | 1.56 (1.33–1.83)* | <0.001 |
| ≥3 | 2.84 (2.48–3.25)* | <0.001 |
Table 3.
|
3-month |
1-year |
2-year |
4-year |
|||||
|---|---|---|---|---|---|---|---|---|
|
Mild |
Severe |
Mild |
Severe |
Mild |
Severe |
Mild |
Severe |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Medical facility type | ||||||||
| Low-volume hospital | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| High-volume hospital | 0.93 (0.83–1.04) | 0.87 (0.77–0.97)* | 0.89 (0.79–1.00)* | 0.87 (0.77–0.97)* | 1.03 (0.91–1.15) | 0.89 (0.79–0.99)* | 1.02 (0.89–1.17) | 0.78 (0.68–0.89)† |
| Surgery type | ||||||||
| No surgery | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Surgery | 1.63 (1.38–1.91)† | 1.15 (1.03–1.29)* | 1.88 (1.60–2.21)† | 1.28 (1.14–1.44)† | 2.25 (1.91–2.64)† | 1.34 (1.19–1.50)† | 1.71 (1.41–2.07)† | 1.46 (1.27–1.68)† |
| Age | ||||||||
| 18–45 years | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 46–59 years | 2.04 (1.45–2.86)† | 1.10 (0.89–1.36) | 1.91 (1.36–2.69)† | 0.96 (0.78–1.19) | 1.84 (1.31–2.59)† | 0.94 (0.76–1.17) | 1.50 (1.01–2.23)* | 0.80 (0.62–1.03) |
| 60–69 years | 3.25 (2.32–4.57)† | 1.34 (1.07–1.67)* | 2.84 (2.02–3.99)† | 1.18 (0.94–1.47) | 2.45 (1.74–3.44)† | 1.05 (0.84–1.32) | 1.59 (1.06–2.38)* | 0.77 (0.59–1.00) |
| ≥70 years | 9.03 (6.53–12.48)† | 1.50 (1.22–1.84)† | 6.55 (4.73–9.08)† | 1.10 (0.90–1.36) | 4.39 (3.16–6.10)† | 0.90 (0.74–1.11) | 2.45 (1.67–3.61)† | 0.72 (0.56–0.92)† |
| Gender | ||||||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 0.80 (0.72–0.88)† | 0.90 (0.81–1.00)* | 0.81 (0.73–090)† | 0.97 (0.88–1.08) | 0.89 (0.81–0.99)* | 1.08 (0.97–1.20) | 0.88 (0.78–0.99)* | 1.03 (0.91–1.18) |
| Arrival mode | ||||||||
| EMS | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| No EMS | 0.86 (0.77–0.97)* | 0.90 (0.74–1.09) | 0.87 (0.77–0.98)* | 0.94 (0.77–1.14) | 0.89 (0.79–1.00)* | 0.93 (0.77–1.13) | 0.90 (0.78–1.03) | 0.94 (0.74–1.18) |
| Medical history | ||||||||
| CCI score | ||||||||
| 0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1 | 1.04 (0.86–1.26) | 0.92 (0.79–1.07) | 1.02 (0.84–1.23) | 0.82 (0.70–0.96)* | 1.03 (0.86–1.25) | 1.03 (0.88–1.20) | 0.99 (0.79–1.23) | 0.96 (0.79–1.16) |
| 2 | 0.96 (0.80–1.15) | 0.79 (0.67–0.93)† | 0.97 (0.81–1.16) | 0.64 (0.55–0.76)† | 0.98 (0.82–1.17) | 0.74 (0.63–0.87)† | 1.05 (0.85–1.30) | 0.68 (0.56–0.83)† |
| ≥3 | 1.29 (1.11–1.49)† | 0.67 (0.58–0.77)† | 1.05 (0.91–1.22) | 0.45 (0.39–0.52)† | 0.95 (0.82–1.10) | 0.48 (0.42–0.55)† | 0.89 (0.75–1.05) | 0.44 (0.38–0.52)† |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download