Abstract
Objective
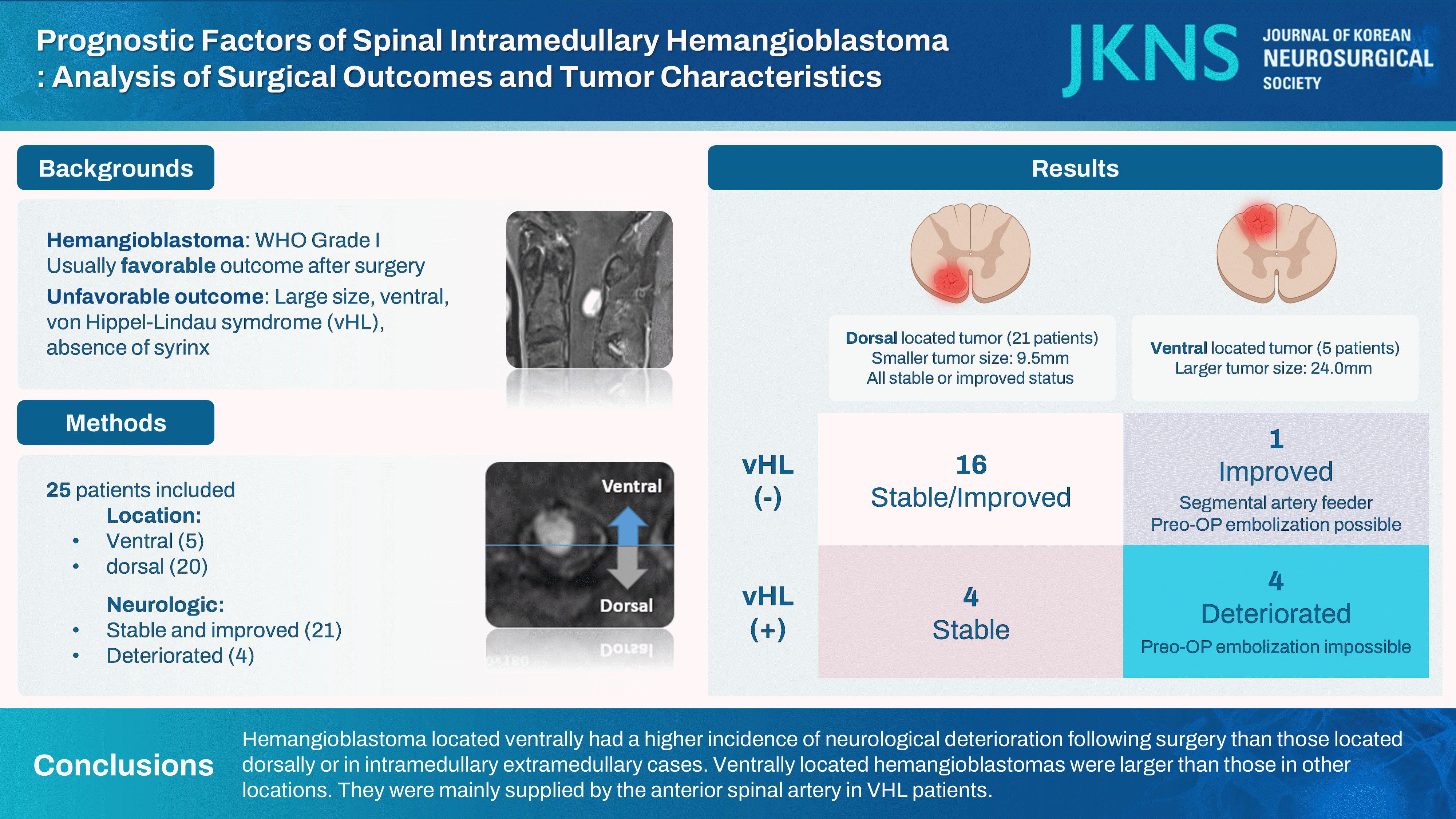
Spinal intramedullary hemangioblastoma is a rare and highly vascularized benign tumor. The characteristics of the tumor, its corresponding location, and surgical outcomes remain unknown. The purpose of this study was to identify risk factors and strategies for neurologic deterioration following hemangioblastoma surgery.
Methods
A comprehensive retrospective analysis was undertaken to evaluate patients who underwent surgical intervention for intramedullary hemangioblastoma at our institution from 1993 to 2022. Patients with at least 1 year of follow-up data were included. The analysis covered patient demographics, pre- and post-operative modified McCormick scale (MMCS), tumor location, and tumor size.
Results
This study included 25 cases. One-year after surgery, neurological deterioration was observed in five cases (20.0%), and neurological improvement was found in nine cases (36.0%). Five cases were ventrally located, and twelve cases were dorsally located. Ventrally located cases were larger in tumor axial size (p=0.029) than dorsal location tumors, resulting in poorer follow-up MMCS and a higher prevalence of von Hippel-Lindau syndrome (VHL) (p=0.042). Three of them were confirmed to be supplied by the anterior spinal artery. In the case of dorsally located cases, there was no neurologic deterioration.
Conclusion
In intramedullary spinal cord hemangioblastomas, cases located ventrally had a higher incidence of neurological deterioration following surgery than those located dorsally or in intramedullary extramedullary cases. Ventrally located hemangioblastomas were larger than those in other locations. They were mainly supplied by the anterior spinal artery in VHL patients.
Spinal intramedullary hemangioblastoma is a highly vascular tumor that accounts for 2% of all central nervous system tumors and 5% of intramedullary spinal cord tumors. It can occur sporadically or in association with von Hippel-Lindau syndrome (VHL), an autosomal dominant genetic disorder characterized by the development of multiple vascular tumor [5,6,9,12,14,19]. Despite being the treatment of choice, surgical management of these tumors remains challenging due to complex anatomy of the spinal cord and the tumor’s intimate relationship with surrounding neural structures [22].
The main objective of surgical intervention is complete tumor resection to relieve cord compression, eliminate the mass of abnormal vascular growth, and ultimately prevent or reverse neurological decline. However, the achievement of this objective is not without potential risks. Due to the tumor’s high vascularization and location within the spinal cord, coagulation during surgery may cause injury to spinal cord tissue itself or lead to coagulation of vital vessels supplying the spinal cord [13,16,29]. Outcomes for patients with hemangioblastoma are typically influenced by factors such as preoperative functional status, large tumor size, ventrally located tumor, peri-tumor syrinx, and the presence of VHL syndrome [10,18]. The location of the tumor within the spinal cord is a crucial yet frequently understudied factor in determining surgical outcomes. It is known that, the location of hemangioblastoma, particularly in relation to anterior spinal artery (ASA) feeders, has a substantial impact on neurological outcomes following surgery [34].
Consecutive patients treated surgically for histologically confirmed spinal hemangioblastoma from January 1993 to July 2021 were retrospectively analyzed after obtaining approval from Gangnam Severance Hospital Institutional Review Board (No. 3-2023-0404). Only patients with postoperative follow-up data for more than 1 year were included. Cases with extramedullary tumors or recurrences at the same site where previous surgery was performed were excluded.
Numerous variables considered to influence surgical outcomes were analyzed, including patient demographics, tumor characteristics, and pre- and post-operative neurological status (immediate post-surgery and 1-, 6-, and 12-month postsurgery). When tumors appeared to originate from ventral vessels of the spinal cord and when most of the tumor was in the anterior half of the spinal cord, the location of the hemangioblastoma was classified as ventral. In contrast, tumors were categorized as dorsal if they originated from dorsal vessels of the spinal cord and if the preponderance of the tumor was in the posterior half of the spinal cord. Interesting tumor characteristics included its size and the presence or absence of a syrinx. Neurological status was evaluated using the modified McCormick scale (MMCS) both before and after surgery : I, intact neurologically, normal ambulation, minimal dysesthesia; II, mild motor or sensory deficit, functional independence; III, modete deficit, limitation of function, independent with external aid; IV, severe motor or sensory deficit, limited function, dependent; V, paraplegia or quadriplegia, even with flickering movement [2]. Change in 12-month post-surgery MMCS were used as an indicator of neurological deterioration or improvement following surgical intervention.
Tumor size was measured as the longest part of the tumor that was enhanced in axial or sagittal view. To determine the extent of the tumor’s cross-section relative to the spinal cord, the following values were measured from the magnetic resonance imaging (MRI) image : tumor axial size and cord axial size (areas of the tumor and the cord including the tumor in the cross-section of the MRI’s T1 enhanced axial image, respectively), tumor/cord ratio (calculated by dividing tumor axial size by cord axial size).
For all statistical analyses, SPSS Statistics 26 (IBM, Chicago, IL, USA) was used. Categorical data were compared using chisquare test or Fisher’s exact test. Mean values were compared using Student’s t-test or Mann-Whitney U test. Probability values less than 0.05 were considered statistically significant.
There were 25 surgeries performed for 23 patients. All surgical procedures were performed using a posterior approach. After executing a laminectomy at the proper level, the dura was opened, and the tumor was removed using appropriate techniques. If the tumors are embedded within the spinal cord, they are accessed via a longitudinal myelotomy. Only one case involved a subtotal resection. Total resection was achieved for the remaining 24 cases. There were no surgical complications, such as postoperative hematoma or cerebrospinal fluid leakage. The average age of patients was 44.4 years (range, 20–80). Females accounted for 68%. Eight cases (32.0%) were genetically confirmed to have VHL syndrome. Four cases (16%) exhibited neurological deterioration 1 year after surgery, while 11 cases (44.0%) exhibited neurological improvement. Twenty cases (80%) were dorsal intramedullary (IM) tumors, and five cases (20.0%) were ventral IM tumors. Syrinx was observed in 24 cases (96.0%) (Table 1). Regardless of the tumor location, the median duration of symptoms was about 12 months.
Before surgery, two (8%) and 18 cases (72%) were categorized as MMCS grade I and grade II, respectively, with most patients exhibiting modest neurological symptoms. In addition, three cases (12%) were categorized as MMCS grade III and two cases (8%) were categorized as MMCS grade IV. Before undergoing surgery, 56%, 20%, 64%, and 8% of patients complained of pain, sensory dysfunction, motor weakness, and sphincter dysfunction, respectively. At 1 year after surgery, nine cases (36%) exhibited neurological improvement, four cases (16.0%) exhibited neurological deterioration, and 12 cases (48.0%) showed no neurological change (Fig. 1).
All four cases with neurological deterioration were ventral IM cases. No case showed postoperative deterioration for cases located dorsally. Cases with neurological deterioration had larger tumor size (10.0 vs. 32.0 mm, p=0.016). They were often diagnosed with VHL (100.0% vs. 19.0%, p=0.009) compared to cases that were neurologically stable or improved. The tumor size was larger (32 vs. 10 mm, p=0.016) and the tumor/cord ratio was higher (0.7±0.3 vs. 0.3±0.2, p<0.001) for neurological deterioration cases. Syrinx was present at a similar proportion (95.2% vs. 100%, p=1) (Table 2).
We noted that most patients with neurologic deterioration had ventrally located IM hemangioblastomas (Table 3). Ventral IM hemangioblastomas had a higher incidence of neurological deterioration (80.0% vs. 0.0%, p=0.001), a larger tumor size (9.5 mm [6.5; 11.5] vs. 24.0 mm [11.0; 40.0], p=0.029), and a higher tumor/cord ratio (0.7±0.2 vs. 0.3±0.2, p=0.001) than dorsal IM. In three cases, the presence of feeders from the ASA posed expected risks, thereby precluding embolization. Each of these three cases was accompanied by neurologic deterioration and a diagnosis of VHL (Fig. 2). One patient, who was pregnant with VHL, was unable to undergo angiography due to radiation exposure risks. After surgery, she showed a worsening of MMCS. In contrast, in one case where a feeder was identified from the anterior radicular artery, embolization was successfully performed. After the operation, this patient's neurological condition improved (Fig. 3).
This study provides a comprehensive analysis of prognostic factors influencing postoperative outcomes of spinal intradural hemangioblastoma, with a particular focus on tumor location within the spinal cord. Dorsal IM cases accounted for 80% of the total cases, no decrease in the MMCS was observed. Studies of dorsally located hemangioblastomas have reported favorable outcomes, with postoperative neurologic deterioration ranging from 0% to 18% [10]. Reportedly, 93% to 100% of hemangioblastomas are located on the dorsal aspect, with more than two-thirds located in the root entry zone of the dorsal root [15]. These tumors are predominantly of pial origin, accumulating around dorsal nerve rootlets or blood vessels. They are frequently encapsulated. Patients with hemangioblastomas located dorsally typically present with sensory changes as initial symptoms, suggesting that these tumors are often diagnosed before they reach a substantial size [21,32]. While numerous studies have indicated that preoperative embolization can be advantageous, our series did not include any cases of dorsal IM that underwent preoperative embolization. However, proper embolization can help preemptively mitigate the risk of intraoperative bleeding. Particularly, embolization of the dorsal artery poses less risk than that of the ASA, offering a distinct advantage [1,28].
In contrast, our cases of ventral hemangioblastomas are typically fed by the ASA. Thus, they require manipulation of the anterior part of the cord for removal, which has been associated with poor surgical outcomes in multiple studies. Characteristics of hemangioblastomas in patients with VHL disease are also referred to as “spinal leptomeningeal hemangioblastomasis”. Although the precise pathogenesis remains unclear, it is known that lesions can occur in atypical locations. Notably, all three patients with ventral hemangioblastomas reported by Van Der Veken et al. [30] were diagnosed with VHL, as were the eight patients with ventral hemangioblastomas studied by Pluta et al. [23]. Ventral hemangioblastomas have been reported to result in worse outcomes in 33% to 80% of cases, likely due to their association with the ASA and the difficulty of surgical access [15,23]. Feeding arteries and drainage veins associated with the tumor are not readily visible in a posterior approach, leading to attempts to use an anterior approach. Pluta et al. [23] have reported better clinical outcomes with an anterior approach to the tumor following corpectomy in eight cases of ventrally located hemangioblastomas. However, Van der Veken et al. [30] have reported the use of a posterior approach for removing ventral hemangioblastomas in three cases, arguing that this approach could avoid motor deficits caused by anterior myelotomy and that it is safer because one ASA supplies two-thirds of the cord’s blood supply, while two posterior spinal arteries supply the remaining third [8,17]. However, all cases in their report involved small-sized tumors accompanied by cysts. Thus, it is uncertain whether their findings could be applied to our cases.
Along with tumor size, VHL, and anterior location, syrinx is one of the factors associated with deterioration that has been studied [10,18]. Although our study showed no association with neurologic deterioration (neurologic stable vs. deterioration : 95.2% vs. 50%, p=0.09), we believe that the presence of peritumoral syrinx around the tumor may be a factor associated with surgical outcome because it facilitates and safely separates the spinal cord from the tumor during surgery.
One of our five cases of ventral hemangioblastoma did not have a feeder from the ASA, making embolization and removal relatively safe. However, feeder from the ASA was present, and embolization could not be performed due to the risk of fatal anterior cord syndrome in the remaining three cases. Interestingly, all patients with feeders from the ASA were diagnosed with VHL disease. In addition, tumor sizes ranged from 11 to 44 mm in tumor axial size, relatively larger than tumors in other locations. This suggests that tumors growing anteriorly might not be detected until they have grown large enough to affect the lateral or posterior tracts responsible for pain or sensory changes. In our series, ventral IM tumors were located between T9 and T12, making it difficult to consider an anterior approach [33]. As with other studies, poor clinical outcomes observed in our study were likely to be influenced by these factors. For ventrally located spinal cord tumors, techniques such as cord rotation by dentate ligamental stay suture and transpedicular approach can be used [11,31]. It is necessary to conduct additional research on these surgical techniques. There are also positive reports on stereotactic radiosurgery for spinal hemangioblastomas, which could be considered an alternative in cases where significant neurological deterioration following surgery is strongly anticipated [4,7,20,24].
In many studies, surgical IM hemangioblastomas is recommended for patients presenting with neurological symptoms [27]. However, there is debate regarding the timing of surgery for asymptomatic sporadic IM hemangioblastomas, with some opinions suggesting that surgical resection should be considered even in the absence of symptoms, while others have recommended surgery once symptoms associated with the lesion emerge [3,25]. For asymptomatic patients with VHL disease who have IM hemangioblastomas, surgery is generally advised when radiological evidence of tumor enlargement is detected [26]. According to our findings, factors such as tumor size and the possibility of preoperative embolization should be considered to predict the outcomes of surgery and assess the benefits of surgical resection in deciding on the timing of the surgery. Additionally, in patients with VHL disease having IM hemangioblastomas, there may be an association with feeders from the ASA, which might not be amenable to embolization. Therefore, it is essential to discuss the potential for neurological deterioration after surgery in advance.
Limitations of this study are the limited number of cases, particularly for ventral hemangioblastomas, the retrospective nature of this study, and potential selection bias. Although it might be difficult to generalize the results of this study due to its small sample size, this research aimed to analyze surgical deterioration cases, providing a deeper understanding of how tumor location could affect surgical outcomes and guide surgical planning and prognosis. To accurately analyze the characteristics and clinical outcomes of ventral spinal hemangioblastomas, multinational and/or multi-institutional research studies are needed. In addition to the advancement of genetic research and treatments, there is a need to investigate genetic therapeutic approaches.
In intramedullary spinal cord hemangioblastomas, cases located ventrally showed more frequent neurological deterioration following surgery than those located dorsally. Ventrally located hemangioblastomas were greater in size than those in other locations. They were mostly supplied by the ASA in VHL patients.
Notes
References
1. Ampie L, Choy W, Khanna R, Smith ZA, Dahdaleh NS, Parsa AT, et al. Role of preoperative embolization for intradural spinal hemangioblastomas. J Clin Neurosci. 24:83–87. 2016.

2. Bellut D, Burkhardt JK, Mannion AF, Porchet F. Assessment of outcome in patients undergoing surgery for intradural spinal tumor using the multidimensional patient-rated core outcome measures index and the modified McCormick scale. Neurosurg Focus. 39:E2. 2015.

3. Boström A, Hans FJ, Reinacher PC, Krings T, Bürgel U, Gilsbach JM, et al. Intramedullary hemangioblastomas: timing of surgery, microsurgical technique and follow-up in 23 patients. Eur Spine J. 17:882–886. 2008.

4. Bridges KJ, Jaboin JJ, Kubicky CD, Than KD. Stereotactic radiosurgery versus surgical resection for spinal hemangioblastoma: a systematic review. Clin Neurol Neurosurg. 154:59–66. 2017.

5. Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 48:55–63. discussion 62-63. 2001.

6. Cristante L, Herrmann HD. Surgical management of intramedullary hemangioblastoma of the spinal cord. Acta Neurochir (Wien). 141:333–339. discussion 339-340. 1999.

7. Daly ME, Choi CY, Gibbs IC, Adler JR Jr, Chang SD, Lieberson RE, et al. Tolerance of the spinal cord to stereotactic radiosurgery: insights from hemangioblastomas. Int J Radiat Oncol Biol Phys. 80:213–220. 2011.

8. Foo D, Rossier AB. Anterior spinal artery syndrome and its natural history. Paraplegia. 21:1–10. 1983.

9. Han B, Zhang L, Jia W. Pediatric spinal hemangioblastomas: clinical features and surgical outcomes of 39 cases. Neurospine. 20:343–352. 2023.

10. Iwasaki Y, Koyanagi I, Hida K, Abe H. Anterior approach to intramedullary hemangioblastoma: case report. Neurosurgery. 44:655–657. 1999.

11. Joshi SS, Moghe VV, Choudhari KA. Spinal cord rotation by denticulate ligamental stay-sutures for anteriorly placed intradural lesions. J Spin Surg. 6:29. 2019.
12. Kageyama H, Tatebayashi K, Yoshimura S, Endo T, Hida K, Mizuno M. Outcomes of intramedullary spinal cord tumor surgery in older versus younger adults: a multicenter subanalysis study by the Neurospinal Society of Japan. Neurospine. 20:678–691. 2023.

13. Lee J, Koyanagi I, Hida K, Seki T, Iwasaki Y, Mitsumori K. Spinal cord edema: unusual magnetic resonance imaging findings in cervical spondylosis. J Neurosurg. 99(1 Suppl):8–13. 2003.

14. Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 361:2059–2067. 2003.

15. Lonser RR, Weil RJ, Wanebo JE, Devroom HL, Oldfield EH. Surgical management of spinal cord hemangioblastomas in patients with von HippelLindau disease. J Neurosurg. 98:106–116. 2003.

16. Malis LI. Atraumatic bloodless removal of intramedullary hemangioblastomas of the spinal cord. J Neurosurg. 97(1 Suppl):1–6. 2002.

17. Manconi M, Mondini S, Fabiani A, Rossi P, Ambrosetto P, Cirignotta F. Anterior spinal artery syndrome complicated by the ondine curse. Arch Neurol. 60:1787–1790. 2003.

18. Mandigo CE, Ogden AT, Angevine PD, McCormick PC. Operative management of spinal hemangioblastoma. Neurosurgery. 65:1166–1177. 2009.

19. Mechtler LL, Nandigam K. Spinal cord tumors: new views and future directions. Neurol Clin. 31:241–268. 2013.
20. Moss JM, Choi CY, Adler JR Jr, Soltys SG, Gibbs IC, Chang SD. Stereotactic radiosurgical treatment of cranial and spinal hemangioblastomas. Neurosurgery. 65:79–85. discussion 85. 2009.

21. Murota T, Symon L. Surgical management of hemangioblastoma of the spinal cord: a report of 18 cases. Neurosurgery. 25:699–707. discussion 708. 1989.

22. Neumann HP, Eggert HR, Scheremet R, Schumacher M, Mohadjer M, Wakhloo AK, et al. Central nervous system lesions in von Hippel-Lindau syndrome. J Neurol Neurosurg Psychiatry. 55:898–901. 1992.

23. Pluta RM, Iuliano B, DeVroom HL, Nguyen T, Oldfield EH. Comparison of anterior and posterior surgical approaches in the treatment of ventral spinal hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 98:117–124. 2003.

24. Ryu SI, Kim DH, Chang SD. Stereotactic radiosurgery for hemangiomas and ependymomas of the spinal cord. Neurosurg Focus. 15:E10. 2003.

25. Sadashivam S, Abraham M, Kesavapisharady K, Nair SN. Long-term outcome and prognostic factors of intramedullary spinal hemangioblastomas. Neurosurg Rev. 43:169–175. 2020.

26. Takai K, Taniguchi M, Takahashi H, Usui M, Saito N. Comparative analysis of spinal hemangioblastomas in sporadic disease and Von Hippel-Lindau syndrome. Neurol Med Chir (Tokyo). 50:560–567. 2010.

27. Takeshima Y, Takami H, Endo T, Mizuno M, Hida K; Investigators of Intramedullary Spinal Cord Tumors in the Neurospinal Society of Japan. Comparison of the recurrence and surgical outcome of spinal hemangioblastoma in sporadic and von Hippel-Lindau diseases: a subanalysis of a nationwide study by the Neurospinal Society of Japan. Neurospine. 20:756–765. 2023.

28. Tampieri D, Leblanc R, TerBrugge K. Preoperative embolization of brain and spinal hemangioblastomas. Neurosurgery. 33:502–505. discussion 505. 1993.

29. Timonin SY, Konovalov NA. Surgical treatment of intramedullary hemangioblastomas: current state of problem (review). Sovrem Tekhnologii Med. 13:83–94. 2021.

30. Van Der Veken J, Gläsker S, Vougioukas V, Van Velthoven V. Posterior approach for anteriorly located cervical spinal cord hemangioblastomas: technical note. J Neurosurg Spine. 29:448–451. 2018.
31. Visco ZR, Liu DD, Leary OP, Oyelese AA, Gokaslan ZL, Camara-Quintana JQ, et al. A transpedicular approach to complex ventrally situated thoracic intradural extramedullary tumors: technique, indications, and multiinstitutional case series. Neurosurg Focus. 50:E19. 2021.

32. Yasargil MG, Antic J, Laciga R, de Preux J, Fideler RW, Boone SC. The microsurgical removal of intramedullary spinal hemangioblastomas. Report of twelve cases and a review of the literature. Surg Neurol. (3):141–148. 1976.
33. Zenner J, Koller H, Hempfing A, Hutter J, Hitzl W, Resch H, et al. Approach-related morbidity in transthoracic anterior spine surgery: a clinical study and review of literature. Coluna/Columna. 9:72–84. 2010.
Fig. 1.
A chart illustrating preoperative, immediate postoperative, and 1-year outcomes. Red arrows represent deterioration. Blue arrows indicate improvement in the modified McCormick scale score. Op : operation, FU : follow-up.
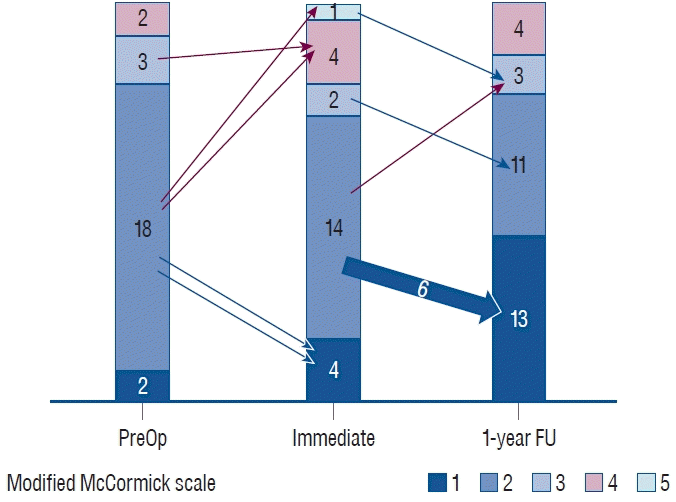
Fig. 2.
A 20-year-old female patient with von Hippel-Lindau syndrome presented with symptoms of pain and weakness in both legs along with sphincter dysfunction. She was diagnosed with a ventral intramedullary hemangioblastoma at T11-12. Her preoperative modified McCormick scale was grade II, which worsened to grade V postoperatively. One year after surgery, it improved to grade III. However, it did not recover to the preoperative level. A : A presyrinx and engorged pial vessels are observed in the T2-weighted image. B and C : A large tumor is observed in T1 contrast-enhanced image, with engorged vessels located anteriorly visible in the axial image. D : Angiography reveals that the feeding artery originated from the anterior spinal artery (arrowhead) with a well-stained tumor (arrow). However, embolization could not be performed.
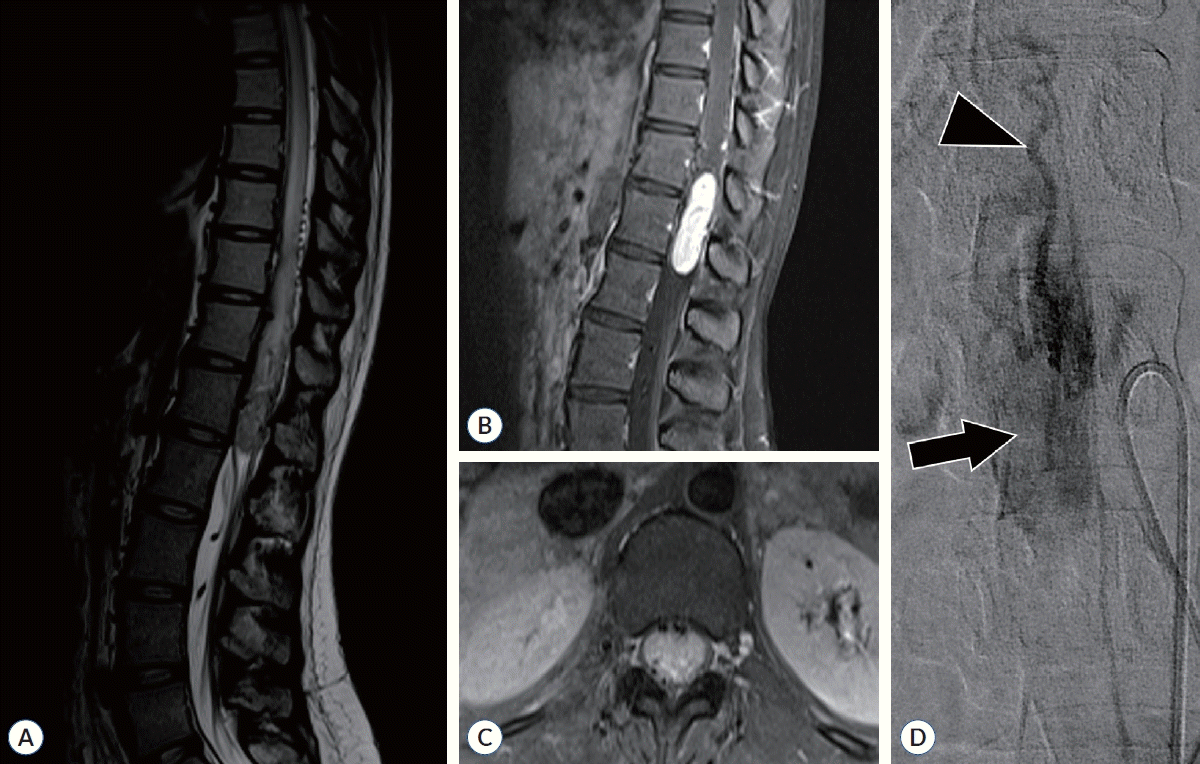
Fig. 3.
A 21-year-old female patient without von Hippel-Lindau syndrome presented with symptoms of pain in both legs, sensory changes, and gait disturbance. She was diagnosed with a ventral intramedullary hemangioblastoma at T8. After undergoing segmental artery embolization, she received surgical treatment. Six months post-surgery, her modified McCormick scale grade improved from II to I. A : A large syrinx encompassing the tumor was observed on T2-weighted image. B and C : T1-weighted image with enhanced contrast. A contrast-enhanced intramedullary tumor located ventrally was observed. D : In angiography, staining of the tumor was obvious (arrowhead). A feeding vessel from the radicular artery at T9-10 into the tumor was identified (arrow), leading to its embolization.
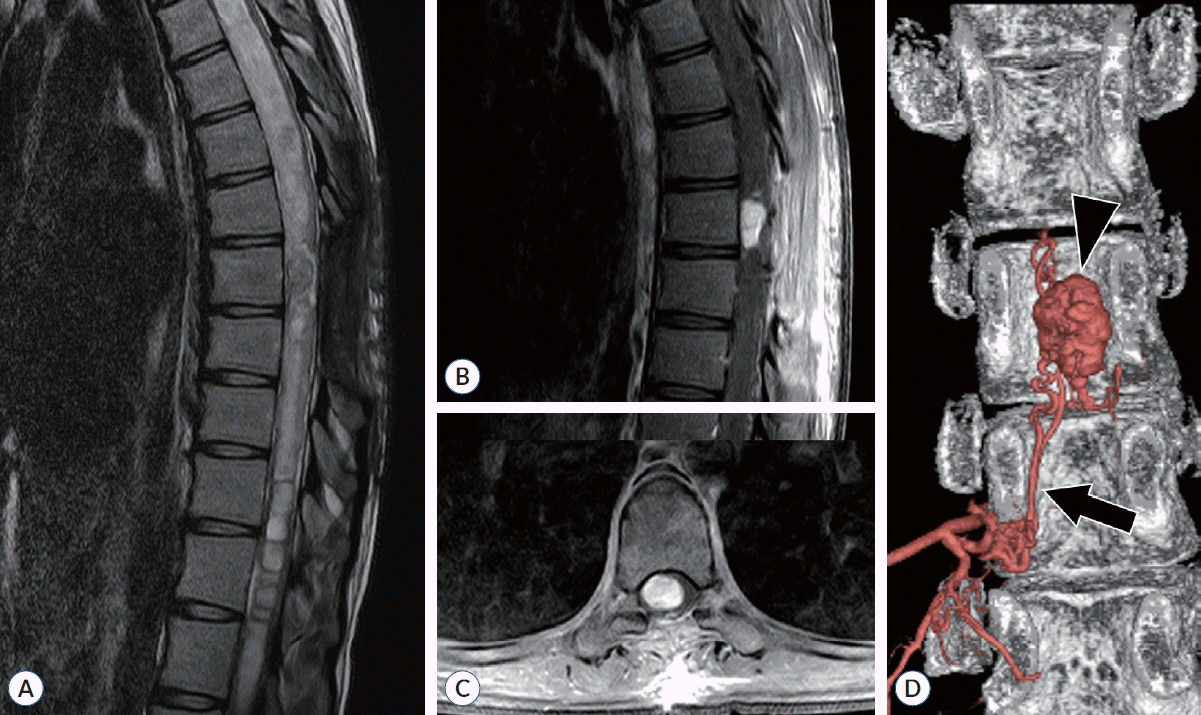
Table 1.
Demographics of cohort population
Table 2.
Comparison of characteristics between neurologic “stable or improved” and “deterioration” with spinal intramedullary hemangioblastoma
| Variable | Stable or improved (n=21) | Deterioration (n=4) | p-value |
|---|---|---|---|
| Sex | 0.797 | ||
| Female | 15 (71.4) | 2 (50.0) | |
| Male | 6 (28.6) | 2 (50.0) | |
| Age (years) | 44.0±16.2 | 46.2±23.8 | 0.815 |
| Operation time (minutes) | 330.0 (240.0–435.0) | 372.5 (267.5–652.5) | 0.824 |
| Estimated blood loss (mL) | 600.0 (500.0–900.0) | 1250.0 (800.0–1650.0) | 0.127 |
| Resection | 0.344 | ||
| Gross total resection | 21 (100.0) | 3 (75.0) | |
| Subtotal resection | 0 (0.0) | 1 (25.0) | |
| Level | 0.195 | ||
| Cervical, C1-C6 | 7 (33.3) | 0 (0.0) | |
| Cervicothoracic, C7-T1 | 1 (4.8) | 0 (0.0) | |
| Thoracic, T2-T8 | 5 (23.8) | 0 (0.0) | |
| Thoracolumbar, T9-L1 | 7 (33.3) | 4 (100.0) | |
| Lumbar, L2-S1 | 1 (4.8) | 0 (0.0) | |
| Tumor location | <0.001* | ||
| Dorsal intramedullary | 20 (95.2) | 0 (0.0) | |
| Ventral intramedullary | 1 (4.8) | 4 (100.0) | |
| Tumor size (mm) | 10.0 (7.0–11.0) | 32.0 (17.5–42.0) | 0.016* |
| Syrinx | 20 (95.2) | 4 (100.0) | 1.000 |
| Duration of symptom (months) | 12.0 (7.0–36.0) | 12.0 (9.0–27.0) | 0.911 |
| VHL | 4 (19.0) | 4 (100.0) | 0.009* |
| Preop MMCS | 1.000 | ||
| 1 | 2 (9.5) | 0 (0.0) | |
| 2 | 15 (71.4) | 3 (75.0) | |
| 3 | 2 (9.5) | 1 (25.0) | |
| 4 | 2 (9.5) | 0 (0.0) | |
| Tumor axial size (cm2) | 0.5±0.3 | 1.4±0.7 | 0.008* |
| Tumor/cord ratio | 0.3±0.2 | 0.7±0.3 | <0.001* |
Table 3.
Comparison of characteristics between dorsal and ventral spinal intramedullary hemangioblastoma
| Variable | Dorsal (n=20) | Ventral (n=5) | p-value |
|---|---|---|---|
| Sex | 1.000 | ||
| Female | 14 (70.0) | 3 (60.0) | |
| Male | 6 (30.0) | 2 (40.0) | |
| Age (years) | 45.1±15.7 | 41.2±23.5 | 0.653 |
| Operation time (minutes) | 320.0 (240.0–437.5) | 370.0 (345.0–400.0) | 0.683 |
| Estimated blood loss (mL) | 625.0 (450.0–900.0) | 1200.0 (500.0–1300.0) | 0.293 |
| Resection | 0.444 | ||
| Gross total resection | 20 (100.0) | 4 (80.0) | |
| Subtotal resection | 0 (0.0) | 1 (20.0) | |
| Level | 0.394 | ||
| Cervical, C1-C7 | 7 (35.0) | 0 (0.0) | |
| Cervicothoracic, C7-T1 | 1 (5.0) | 0 (0.0) | |
| Thoracic, T1-T8 | 4 (20.0) | 1 (20.0) | |
| Thoracolumbar, T9-L1 | 7 (35.0) | 4 (80.0) | |
| Lumbar, L2-S1 | 1 (5.0) | 0 (0.0) | |
| Tumor size (mm) | 9.5 (6.5–11.5) | 24.0 (11.0–40.0) | 0.029* |
| Syrinx | 19 (95.0) | 5 (100.0) | 1.000 |
| Duration of symptom (months) | 12.0 (7.5–36.0) | 12.0 (6.0–12.0) | 0.533 |
| VHL | 4 (20.0) | 4 (80.0) | 0.042* |
| Aggravated | 0 (0.0) | 4 (80.0) | 0.001* |
| Improved | 8 (40.0) | 1 (20.0) | 0.755 |
| Preop MMCS | 0.708 | ||
| 1 | 2 (10.0) | 0 (0.0) | |
| 2 | 14 (70.0) | 4 (80.0) | |
| 3 | 2 (10.0) | 1 (20.0) | |
| 4 | 2 (10.0) | 0 (0.0) | |
| Tumor axial size (cm2) | 0.5±0.3 | 1.3±0.6 | 0.053 |
| Tumor/cord ratio | 0.3±0.2 | 0.7±0.2 | 0.001* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download