Abstract
Background and Objectives
Chronic rhinosinusitis (CRS) is an inflammatory disease which has a complex etiopathogenesis and can be a significant burden on the patient and health systems. Based on European Position Paper on Rhinosinusitis and Nasal Polyps, peripheral blood eosinophil and total serum IgE are markers of inflammation type 2 CRS endotype. However, there is a marked global heterogeneity in inflammatory endotypes among patients with CRS without nasal polyps (CRSsNP) or CRS with nasal polyps (CRSwNP) among the Asians and Caucasians with CRS. To determine the concurrence between CRS endotypes biomarkers with peripheral blood eosinophil, eosinophil-neutrophil ratio (ENR), and total serum immunoglobulin E (IgE) in the diagnosis of CRS.
Subjects and Method
This study used an observational analytic study with a cross-sectional design, with total of 26 patients were included in this study. Examination of endotype biomarkers was carried out using the enzyme-linked immunosorbent assay method and peripheral blood eosinophil, ENR, and total serum IgE were taken from routine hematological examinations.
Results
We found a significant relationship between ENR values and CRS endotypes (p= 0.021); however, there was no relationship found between the values of peripheral blood eosinophil and total serum IgE with CRS endotypes (p>0.05). The ENR value ≥0.06 has a 6.14 times greater risk of developing type 2 inflammation (95% confidence interval 1.64-23; p<0.001).
Chronic rhinosinusitis (CRS) is an inflammatory disease of the nasal cavity and paranasal sinuses mucosa that has complex etiopathogenesis and can cause significant physical, emotional and economic burdens for patients and health system [1]. Based on our research at Dr. Saiful Anwar general hospital in Indonesia, CRS cases requiring surgery account for around 38% of all patients admitted to the rhinology division [2]. In recent years, many attempts have been made to describe CRS in terms of endotypes, each defined by different molecular mechanisms and identified by matching biomarkers [1,3].
Based on integrated care pathway European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2020 for primary diffuse CRS, to differentiate between type 2 or non-type 2 endotypes, laboratory examinations are required. Type 2 endotype is indicated by increased immunoglobulin E (IgE) and eosinophilia [1]. Studies have suggested that environmental and genetic factors can also influence inflammatory endotypes in CRS. There is a marked global heterogeneity in inflammatory endotypes among patients with CRS without nasal polyps (CRSsNP) or CRS with nasal polyps (CRSwNP) among the Asian and Caucasian CRS [4], so we need to determine whether the criteria of clinical endotype of CRS listed in EPOS can be applied in our patients. With the development of biologic agents, especially in the cases of “difficult to treat” CRS, the differentiation between endotypes clinically becomes imperative. The implementation of endotype-based therapy is expected to lead to better disease management.
It is known that peripheral blood eosinophils and total serum IgE can be potential markers of type 2 inflammatory endotype CRS, although the results are various [1,4,5]. The eosinophil-neutrophil ratio (ENR), which is the ratio of peripheral blood eosinophil derivatives, is a new biomarker of systemic inflammation that shows a comparison between eosinophil and neutrophil values [6]. ENR values have been widely studied in relation to eosinophilic asthma and allergic rhinitis [7], but their role in CRS is still limited. ENR can show the dominance of inflammation cells without having to determine the exact cut off point in asserting the main inflammation cells in CRS. Thus, the main focus of this study was to analyze the relationship between CRS endotype and peripheral blood eosinophil values, ENR, and total serum IgE in patients diagnosed with CRS.
This was a cross-sectional analytic observational study between December 2021-October 2022 in Otorhinolaryngology Head and Neck Surgery Department. This study was approved by the Saiful Anwar General Hospital ethical committee (No. 400/025/K.3/102.7/2023). The inclusion criteria were patients diagnosed with CRS based on EPOS 2020 with age ≥18-years old without previous history of nasal or sinus surgery. Patients without any other diseases such as eosinophilic granulomatosis with polyangiitis, sinonasal tumor, cystic fibrosis, primary ciliary dyskinesia, autoimmune, and hematologic diseases, CRS which does not only involve the posterior group of paranasal sinuses, does not use systemic or local corticosteroids and/or systemic antibiotics within 4 weeks before Functional Endoscopic Sinus Surgery (FESS), and the patient had laboratories tested for complete blood count, differential count, and total serum IgE 30 days before FESS included in this study. The patients who do not meet the inclusion criteria are being excluded as the subjects of this research. The control groups are adult patients aged ≥18 years, diagnosed with nasal turbinate hypertrophy and septal deviation and eligible for surgical intervention (e.g., turbinoplasty, septoplasty). Patients with allergic rhinitis and patients who used corticosteroids or antibiotics within 4 weeks before specimen collection were excluded from the control group of this study. The control group in this study was only to determine the primary CRS endotype within the study group.
A tissue biopsy of the patient’s uncinate process mucosa was taken by uncinectomy using backbiter forceps under general anesthesia. The uncinate process mucosal tissue is put in a cooler box and then sent to the Biomedical Laboratory at the Faculty of Medicine Brawijaya University, or frozen in a refrigerator with a temperature of -80°C if the sample delivery is more than 24 hours. For the controls, the tissue was collected from septal or turbinate mucosa as part of the treatment procedure and sent to the laboratory with the same tissue handling procedure as the uncinate process.
Enzyme-linked immunosorbent assay (ELISA) examination of the mucosal endotype begins with tissue preparation and weighing. The tissue was homogenized using the PRO-PREPTM (17081; iNtRON Biotechnology, Inc., Seongnam, Korea). In determining the endotype, this study used the same biomarkers as those used in the study by Stevens, et al. [8]. Type 1 was characterized by the detection of interferon gamma (IFN-γ), type 2 Charcot Leyden crystal (CLC) and/or eosinophilic cationic protein (ECP), while type 3 interleukin (IL)- 17A. The levels of the biomarker proteins ECP (EH1916) and CLC (EH1340) for type 2, IFN-γ (AQ-H0164-B) for type 1, and IL-17A (EH3267) for type 3 were measured using the FineTest ELISA kit (Wuhan Fine Biotech Co., Ltd., Wuhan, China). The optical density absorbance at 450 nm was read using ZENIX-320 (ZENIX, South Tangerang, Indonesia) microplate reader. Inflammation types determine by tissue protein levels more than 90 percentile of control tissue.
All patients had a blood sample taken approximately 1 month before FESS to obtain their eosinophil, neutrophil, and total serum IgE and their ENR were calculated. The peripheral blood eosinophil and ENR examination was done using XN1000TM Hematology Analyzer (Sysmex, Kobe, Japan), meanwhile total serum IgE examination using the Chemi Luminescent Immunosorbent Assay method with Cobas e411 Analyzer (Roche, City, Germany). All assays were performed at the same laboratory (Central Laboratory, Saiful Anwar General Hospital).
The association analysis between CRS endotypes (type 2 and non-type 2) as categorical dependent variables with peripheral blood eosinophil, ENR, and total serum IgE as numerical independent variables, was analyzed by using an independent t-test or Mann-Whitney test. The cut-off points of peripheral blood eosinophil values, ENR, and total serum IgE were determined by the receiver operating characteristic (ROC) test. After obtaining the cut-off points, to analyze the risk of endotype in primary CRS (type 2 or non-type 2) a chi-squared test was performed, and the strength of the association was presented in the form of a prevalence ratio. A 95% confidence interval (CI) with p-value <0.05 was considered to be statistically significant. All analysis was performed with SPSS version 25 for Mac (IBM Corp., Armonk, NY, USA).
A total of 26 patients with CRS was included in this study with the age ranged from 18 to 62 years old with a mean age of 36.08±3.0 years. There were 15 female (57.7%) and 11 male (42.3%). The main symptoms observed among the patients were nasal obstruction for 61.5%, with the mean duration of symptoms was 105.15±23.6 weeks. The mean of Sino-nasal Outcome Test (SNOT-22) total score was 32.35±8.3 with the highest score observed from type 2 endotypes CRS for 35.0±8.5. It was found that the number of CRS patients with and without nasal polyps was the same, that is 50%. The demographic and clinical characteristics details can be seen in Table 1.
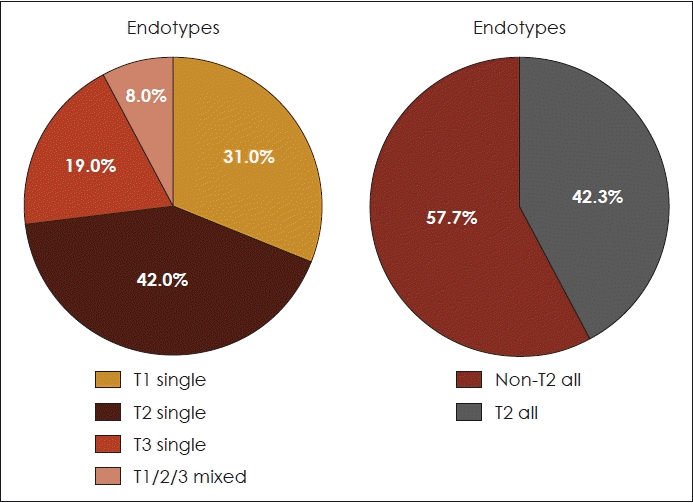
Eight samples of sinonasal mucosa were obtained as controls, consisting of 5 patients with septal deviation who underwent septoplasty, and 3 patients with turbinate hypertrophy who underwent turbinoplasty. The biomarker protein level of the primary CRS sample is considered to be positive if it is higher than the 90th percentile of the biomarker protein levels in all control tissues. The 90th percentile of control tissue biomarker protein levels was 0.685 ng/mL for CLC, 380.743 pg/mL for ECP, 19.297 pg/mL for IFN-γ, and 48.36 pg/mL for IL-17A. Of the 26 samples of primary CRS patients, the percentage of types 1, 2, and 3, which were found single, were 31.0%, 42.0%, and 19.0%, while the remaining mixed endotype, namely type 1/2/3 was 8.0%. There are 3 samples with a mixed type and further recalculation was performed to determine the most dominant type based on the highest percentage difference between the biomarker and control level. The final result for the pattern of primary CRS endotype in study samples could be grouped into type 2 (42.3%) and non-type 2 (57.7%). The detail can be seen in Fig. 1.
An independent T-test was performed on percentage and absolute peripheral blood eosinophil values, as well as total serum IgE with CRS inflammatory endotype, we obtained that there was no statistically significant mean difference (p>0.05). The ENR values were analyzed using the Mann-Whitney test and statistically there was a significant mean difference (p=0.021) between the ENR values of the type 2 and non-type 2 inflammatory endotype groups. The result of correlation analysis can be seen in Table 2.
The ROC analysis showed the optimal cut-off value of ENR is 0.06 with area under curve (AUC) 76.4% (95% CI 54.7%-98.0%; p=0.024), can be seen on Fig. 2. ENR values ≥0.06 categorized as eosinophil dominance/type 2 and ENR <0.06 categorized as neutrophil dominance/non-type 2. Risk analysis of the CRS endotypes was carried out using the chi-square test with the results showed that ENR value ≥0.06 has possibility 6.14 times higher for experiencing CRS inflammatory endotype type 2, compared to with non-type 2 (95% CI 1.64-23; p<0.001).
The study found that CRS was found to be affected patients in their productive age with the age range between 18 to 65 years old [1]. Our research found a similar mean age of patients affected with CRS for 36.08±3.0 years old. With the increase in age, the production of the S100 family protein was decreased which causing the cell proliferation, repair, and epithelial defense to be impaired and lead to the increased risk of abnormal microbial colonization following the chronic inflammation [9]. In this study, we found that the prevalence of CRS was more dominant in female (57.7% vs. 42.3%). CRS prevalence according to gender as a whole is still a debate, but epidemiology studies reported female tend to experience CRS two times higher rather than males [1,2,10], meanwhile other studies found higher prevalence on males [11]. From the available data, heterogeneity of sex prevalence in CRS cases was obtained which was influenced by several factors such as environmental, hormonal, and geographical [10,11]. According to EPOS 2020 [1], the prevalence of cardinal symptoms in CRS patients with and without polyps who are going to undergo surgery, the most common complaints are nasal blockage/congestion and the lowest complaints of facial pain are in CRSwNP [1]. In this study, similar results were obtained, complaints of nasal blockage/congestion were more dominant in non-type 2 endotypes, while headaches were lowest in type 2 endotypes. The SNOT-22 is now regarded as the most appropriate instrument in the evaluation of health-related quality-of-life impairment in CRS patients [1]. The SNOT-22 can be stratified into ‘mild’ being defined on the SNOT-22 score as 8-20, ‘moderate’ as >20-50 and ‘severe’ as >50 [1,12]. The overall severity rating of symptoms is obviously highly dependent upon the population being studied [1,12]. Patients in secondary care awaiting surgery report mean symptom severity scores in the moderate to severe range [1,12], similar results were obtained in this study with the mean total score 32.35±8.3, which is included in the moderate severity level. In this study we obtained nasal polyp more prevalent on type 2 CRS endotype. However, it should be emphasize that not all of nasal polyps are related or equals with type 2 inflammation and vice versa.
In determining the endotype, this study used the same biomarkers as those used in the study by Stevens, et al. [8]. Type 1 was characterized by the detection of IFN-γ, type 2 CLC, and/or ECP, while type 3 IL-17A. Based on their study, there was a higher percentage of type 2 than non-type 2 [8]. In contrast, our study finds that there was a higher percentage of non-type 2 than type 2. These suggested that there was a difference in environmental factors and genetic influences between countries that are thought to be the basis for variations in the types of mucosal inflammation in CRS [4,8,13,14].
This study determining the endotype without using the typical definition of CRS based on the eosinophilic count. The classification of type 2 CRS is characterized by eosinophilic inflammation, whereas non-type 2 is neutrophilic [15]. However, the definition of CRS based on the eosinophilic count might be prone to bias and not reflecting the actual activation of eosinophils in CRS. Moreover, the protein biomarkers such as ECP, major basic protein and eotaxin were considered to be more accurate in determining the degranulated or eosinophil infiltration compared to eosinophilic count [16]. This is also supported by other study conducted by Maharani, et al. [17] showing that there was no significant association between ECP endotype biomarker and the type of tissue inflammation based on predetermined cut-offs. The ECP hold a potential as a biomarker for type 2 for its association with eosinophilic type of inflammation. We propose using ECP as the main biomarker to identify type 2 CRS. The CRS endotyping based on the tissue inflammation response, which may be influenced by various factors, should be replaced with more specified biomarkers [17].
The gold standard for establishing a CRS endotype is by analyzing the gene or protein expression in the patient’s sinonasal tissues. Several genes and proteins have been studied which are the best markers for identifying the type of mucosal inflammation of CRS [4,8,13]. The use of these biomarkers is very limited due to invasive sampling, biomarker measurements that are not included in routine investigations in hospitals, and high costs that will be incurred by patients before surgery. Therefore, establishing the endotype using other practical parameters is currently the focus of CRS research, such as this study.
In this study, there was no statistically significant difference in peripheral blood eosinophil count (p>0.05) between endotypes, but there was a tendency for the percentage of eosinophils in the peripheral blood to be higher in type 2 endotypes. Unlike the result of our research, others have evidence that suggests that peripheral blood eosinophils are a relevant and reliable biomarker for eCRS with or without nasal polyps. However, it’s use as a replacement marker for tissue eosinophilia remains limited [4,5,18]. Gitomer, et al. [19] found no correlation between blood eosinophils and tissue eosinophils, because local eosinophil actions at the tissue level such as activation and migration often occur without an increase in blood eosinophils [19]. Peripheral blood eosinophil counts may also be artificially increased by parasitic infections, comorbid allergies, autoimmune disorders, or drug side effects [20]. In addition, because the nose is a small organ with minimal impact on the blood compartment, there is a high risk of an undiagnosed type 2 immune reaction on the basis of haematological parameters alone [20].
Many studies have been carried out on the ratio of blood parameters by various researchers [6,21,22]. The ENR, which is the ratio of peripheral blood eosinophil derivatives, is a new biomarker of systemic inflammation that shows a comparison of eosinophil and neutrophil values [6]. ENR values have been widely studied related to eosinophilic asthma [7] and allergic rhinitis [23,24], but their role in CRS is still limited. Study on ENR in CRS with using nasal polyp (NP) tissue samples was already conducted by Golebski, et al. [25] and Shaghayegh, et al. [26] Previous studies has found that NP on CRS patients are characterized by an eosinophilic inflammation with high tissue eosinophilia, high IL-5 and IgE, Th2-polarized responses, and an enrichment of ILC2s [25]. Golebski, et al. found a positive correlation between ILC2s and the ENR in NP, suggesting that ILCs are involved in the recruitment of eosinophils and neutrophils and that ILC2s in CRSwNP activate eosinophils. Other study by Shaghayegh, et al. [26] found that NP or mucosal samples had eosinophils to neutrophils ratio that significantly higher in CRSwNP patients compared to CRSsNP (p<0.0001) and controls (p=0.0009). This finding, indicates the presence of an ongoing immune response dominated by type-2 inflammation [26]. However, study on ENR in CRS using blood samples was first studied by Bayer, et al. [6] studied whether hematological indices of the peripheral blood are associated with revision surgery in patients with CRS undergoing endoscopic sinus surgery (ESS) and found by univariate analysis that increasing ENR values were associated with high ESS revision procedure (p<0.001) [6].
Based on previously reported studies, we are interested in examining the blood ratio of eosinophils and neutrophils in CRS and see its relationship with type 2 and non-type 2 CRS endotypes based on the inflammatory biomarkers CLC, ECP, IFN-γ, and IL-17A. The present study of ours is the first one to report the possible relationship between CRS endotypes based on EPOS 2020 (type 2 and non-type 2) using biomarkers of type 2 inflammation (CLC and ECP) and non-type 2 (IFN-γ, IL-17) with the blood eosinophils-neutrophils ratio. Significant results on the ENR value in this study, can also be explained that the variable measurement level with a ratio is a better measurement value than relative numeric value. The ratio can show how big or small a quantity is when compared to the others, in this case the proportion of eosinophil values increases when compared to the neutrophil values in patients with type 2 endotype CRS. It can be concluded that the ENR value can be an indicator/index of peripheral blood of eosinophil derivatives beneficial in type 2 endotype CRS.
In this study, there was no statistically significant mean difference (p>0.05) between total serum IgE and CRS endotypes. It is widely understood that locally produced IgE is a more potent pathophysiological cause of disease than systemic IgE in CRS [27-29]. It is hypothesized that this is due to local class switching to IgE in the pathogenesis of CRS and not from the systemic circulation. Total serum IgE, positive skin prick test, or previous immunoassay for serum specific IgE did not show a significant association with elevated tissue IgE [29]. Local class switching to IgE and local IgE production have also been shown to be associated with Staphylococcus aureus which plays an important role as a disease modifier in CRSwNP and asthma, by releasing enterotoxins. Staphylococcus aureus enterotoxin (SE) has been shown to act as a superantigen that activates T-cells polyclonally, releases Th2 cytokines and amplifies eosinophilic inflammation, and B-cells can induce polyclonal IgE and immunoglobulin G (IgG)/IgG4 production. Other effects of SE on local inflammation are inhibition of Treg cells, decreased eosinophil apoptosis, and induction of chemokines from epithelial cells [30]. Research by Zhang, et al. [30] found that tissue mast cell reactivity in CRS patients with nasal polyps when exposed to allergens and the presence of specific IgE against inhalant allergens or Staphylococcal enterotoxin B (SEB) was associated with tissue, but not with serum. Polyclonal IgE induced by the SEB superantigen contributes to persistent inflammation by continuously activating mast cells [30].
The difference in the results of this study with other studies can also be related to differences in inflammatory endotypes in Indonesia and around the world [4,8,13,14]. The environmental and geographical influences shown by the diversity of inflammatory endotypes in various countries play a role in increasing the complexity of diagnosis and implementation of endotype-based CRS therapy. How environmental, genetic factors, or a combination of both may affect the development of type 1, type 2, or type 3 inflammatory endotypes, will ultimately influence the role of endotype association with blood eosinophils, ENR, and total serum IgE.
The limitation of this study is our samples are CRS cases with more severe inflammation who had failed to respond to medication. We don’t have the data in those who had less severe inflammation and does not require surgery. As a result, the conclusions from this study are based on a patient population with moderate to severe disease. For the direction we want to conduct similar research that includes outpatient CRS with a larger number of samples.
In conclusion, there’s a relationship between ENR values and CRS endotypes, meanwhile no relationship was found between values of peripheral blood eosinophil and total serum IgE with CRS endotypes. ENR value ≥0.06 can be used in screening and diagnosing clinically of endotype type 2 of CRS.
ACKNOWLEDGMENTS
The authors provide equal contribution in patient’s examination, diagnostic procedure, treatment, and writing the original article.
Notes
Author contributions
Conceptualization: Iriana Maharani, Widia Isa Aprillia Sujana. Data curation: all authors. Formal analysis: all authors. Funding acquisition: all authors. Investigation: all authors. Methodology: all authors. Project administration: all authors. Resources: all authors. Software: all authors. Supervision: all authors. Validation: all authors. Visualization: all authors. Writing—original draft: Iriana Maharani, Widia Isa Aprillia Sujana. Writing—review & editing: Iriana Maharani, Widia Isa Aprillia Sujana.
REFERENCES
1. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; 58(Suppl S29):1–464.
2. Maharani I, Wijaya J. Chronic rhinosinusitis epidemiology study in those who need a surgical intervention at Saiful Anwar General Hospital Ward January 1st, 2018–December 31th 2021. Egypt J Ear Nose Throat Allied Sci. 2023; 24(24):1–6.
3. Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol. 2019; 122(1):33–40.

4. Staudacher AG, Peters AT, Kato A, Stevens WW. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020; 124(4):318–25.

5. Ho J, Hamizan AW, Alvarado R, Rimmer J, Sewell WA, Harvey RJ. Systemic predictors of eosinophilic chronic rhinosinusitis. Am J Rhinol Allergy. 2018; 32(4):252–7.

6. Bayer K, Hamidovic S, Brkic FF, Besser G, Mueller CA, Liu DT. Peripheral eosinophil count and eosinophil-to-lymphocyte ratio are associated with revision sinus surgery. Eur Arch Otorhinolaryngol. 2023; 280(1):183–90.

7. Zhang XY, Simpson JL, Powell H, Yang IA, Upham JW, Reynolds PN, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy. 2014; 44(9):1137–45.

8. Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2019; 7(8):2812–20.e3.

9. Renteria AE, Mfuna Endam L, Desrosiers M. Do aging factors influence the clinical presentation and management of chronic rhinosinusitis? Otolaryngol Head Neck Surg. 2017; 156(4):598–605.

10. Ference EH, Tan BK, Hulse KE, Chandra RK, Smith SB, Kern RC, et al. Commentary on gender differences in prevalence, treatment, and quality of life of patients with chronic rhinosinusitis. Allergy Rhinol (Providence). 2015; 6(2):82–8.

11. Brescia G, Contro G, Ruaro A, Barion U, Frigo AC, Sfriso P, et al. Sex and age-related differences in chronic rhinosinusitis with nasal polyps electing ESS. Am J Otolaryngol. 2022; 43(2):103342.

12. Maharani I, Sujana WIA. The correlation between the CT-scan scoring system and the SNOT-22 score in adult chronic rhinosinusitis. Egypt J Ear Nose Throat Allied Sci. 2022. (23):1–4.
13. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; 138(5):1344–53.
14. Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017; 139(2):699–703.e7.

15. Ahern S, Cervin A. Inflammation and endotyping in chronic rhinosinusitis—a paradigm shift. Medicina (Kaunas). 2019; 55(4):95.

16. Lou H, Zhang N, Bachert C, Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol. 2018; 8(11):1218–25.

17. Maharani I, Intan M, Indrasworo D, Anita KW. The correlation of tissue’s endotype biomarkers and dominant inflammation cell in chronic rhinosinusitis. Korean J Otorhinolaryngol-Head Neck Surg. In press 2023.

18. Marcus S, Roland LT, DelGaudio JM, Wise SK. The relationship between allergy and chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. 2018; 4(1):13–7.

19. Gitomer SA, Fountain CR, Kingdom TT, Getz AE, Sillau SH, Katial RK, et al. Clinical examination of tissue eosinophilia in patients with chronic rhinosinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2016; 155(1):173–8.

20. Fadda GL, Galizia A, Galizia G, Castelnuovo P, Bignami M, Cavallo G. Multiparametric analysis of factors associated with eosinophilic chronic rhinosinusitis with nasal polyps. Ear Nose Throat J. 2022; 101(6):NP256–62.

21. Brescia G, Parrino D, Zanotti C, Tealdo G, Barion U, Sfriso P, et al. Blood eosinophil and basophil values before and after surgery for eosinophilic-type sinonasal polyps. Am J Rhinol Allergy. 2018; 32(3):194–201.

22. Brescia G, Sfriso P, Marioni G. Role of blood inflammatory cells in chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 2019; 139(1):48–51.

23. Kant A, Terzioğlu K. Association of severity of allergic rhinitis with neutrophil-to-lymphocyte, eosinophil-to-neutrophil, and eosinophil-to-lymphocyte ratios in adults. Allergol Immunopathol (Madr). 2021; 49(5):94–9.
24. Selçuk A, Keren M. The evaluation of eosinophil-to-lymphocyte, eosinophil-to-neutrophil, and neutrophil-to-lymphocyte ratios in adults with allergic/non-allergic rhinitis. Gulhane Med J. 2023; 65(1):51–5.

25. Golebski K, Ros XR, Nagasawa M, van Tol S, Heesters BA, Aglmous H, et al. IL-1β, IL-23, and TGF-β drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat Commun. 2019; 10(1):2162.

26. Shaghayegh G, Cooksley C, Bouras G, Houtak G, Nepal R, Psaltis AJ, et al. S. aureus biofilm metabolic activity correlates positively with patients’ eosinophil frequencies and disease severity in chronic rhinosinusitis. Microbes Infect. 2023; 25(8):105213.

27. Dennis SK, Lam K, Luong A. A review of classification schemes for chronic rhinosinusitis with nasal polyposis endotypes. Laryngoscope Investig Otolaryngol. 2016; 1(5):130–4.

28. Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007; 137(6):925–30.

Fig. 2.
Recivier operating characteristic (ROC) curve eosinophilneutrophil ratio. Diagonal segments are produced by ties.
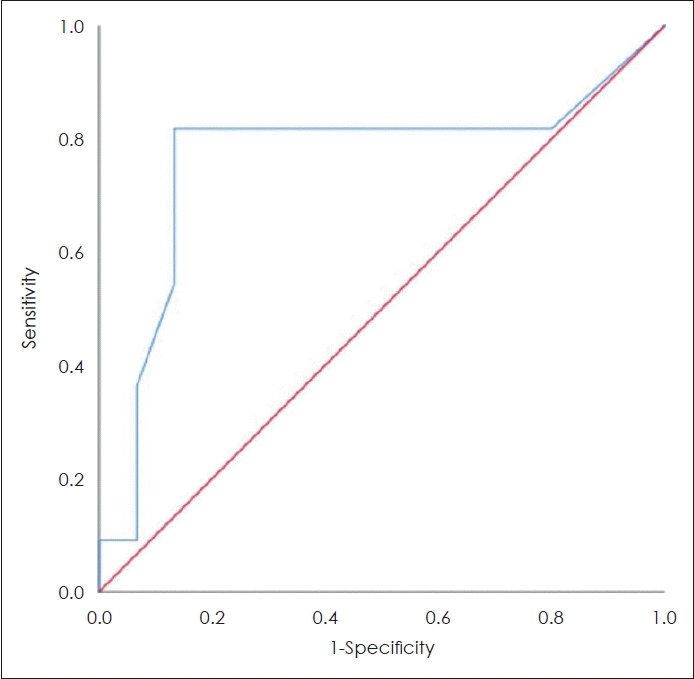
Table 1.
Demographic and clinical characteristic of patients with chronic rhinosinusitis
| Variables | All subject (n=26) |
Endotypes |
p | |
|---|---|---|---|---|
| Type-2 (n=11) | Non-type 2 (n=15) | |||
| Age | 36.08±3.0 | 35.36±5 | 36.6±3.85 | 0.603‡ |
| Sex | 0.246† | |||
| Male | 11 (42.3) | 3 (11.5) | 8 (30.8) | |
| Female | 15 (57.7) | 8 (30.8) | 7 (26.9) | |
| Main symptom | ||||
| Nasal blockage/obstruction/congestion (ref) | 16 (61.5) | 7 (31.8) | 9 (40.9) | |
| Nasal discharge | 6 (23.1) | 3 (13.6) | 3 (13.6) | >0.999† |
| Facial pain/pressure | 4 (15.4) | 1 (5.0) | 3 (15.0) | 0.619† |
| Reduction/loss of smell | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Duration of symptom (weeks) | 105.15±23.6 | 122.82±39.6 | 92.2±29.5 | 0.309‡ |
| Total score SNOT-22 | 32.35±8.3 | 35.0±8.5 | 30.4±7.8 | 0.166ǁ |
| Nasal polyps | 0.047*§ | |||
| With | 13 (50.0) | 8 (30.8) | 5 (19.2) | |
| Without | 13 (50.0) | 3 (11.5) | 10 (38.5) | |
Table 2.
Analysis of chronic rhinosinusitis endotype with hematologic biomarkers (n=26)
| Variables |
Endotypes |
p | |
|---|---|---|---|
| Type-2 (n=11) | Non-type 2 (n=15) | ||
| Blood eosinophils (%) | 0.094† | ||
| Mean±SD | 4.6±1.14 | 2.9±0.9 | |
| Median (min-max) | 4.3 (0.7-15) | 1.7 (0.3-11.5) | |
| Blood eosinophils (absolute) | 0.152† | ||
| Mean±SD | 0.3±0.075 | 0.23±0.08 | |
| Median (min-max) | 0.24 (0.05-0.98) | 0.12 (0.02-1.09) | |
| Eosinophil-neutrophil ratio | 0.021*‡ | ||
| Mean±SD | 0.09±0.03 | 0.04±0.01 | |
| Median (min-max) | 0.07 (0.0-0.33) | 0.02 (0.0-0.21) | |
| Total serum immunoglobulin E | 0.162† | ||
| Mean±SD | 324.05±117.24 | 675.5±575.4 | |
| Median (min-max) | 130.9 (24-1155) | 54.35 (6.88-8718) | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download