1. Baloch ZW, Livolsi VA. Follicular-patterned lesions of the thyroid: the bane of the pathologist. Am J Clin Pathol. 2002; 117:143–50.
2. Zacks JF, de las Morenas A, Beazley RM, O’Brien MJ. Fine-needle aspiration cytology diagnosis of colloid nodule versus follicular variant of papillary carcinoma of the thyroid. Diagn Cytopathol. 1998; 18:87–90.

3. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017; 27:1341–6.

4. Deveci MS, Deveci G, LiVolsi VA, Baloch ZW. Fine-needle aspiration of follicular lesions of the thyroid: diagnosis and follow-up. Cytojournal. 2006; 3:9.
5. Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: an experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012; 40:399–403.

6. Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007; 111:306–15.

7. Bommanahalli B, Bhat R, Rupanarayan R. A cell pattern approach to interpretation of fine needle aspiration cytology of thyroid lesions: a cyto-histomorphological study. J Cytol. 2010; 27:127–32.

8. Bongiovanni M, Sykiotis GP, La Rosa S, et al. Macrofollicular variant of follicular thyroid carcinoma: a rare underappreciated pitfall in the diagnosis of thyroid carcinoma. Thyroid. 2020; 30:72–80.

9. Na HY, Moon JH, Choi JY, et al. Preoperative diagnostic categories of fine needle aspiration cytology for histologically proven thyroid follicular adenoma and carcinoma, and Hurthle cell adenoma and carcinoma: analysis of cause of under- or misdiagnoses. PLoS One. 2020; 15:e0241597.

10. Vuong HG, Ngo HT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: a systematic review and meta-analysis. Cancer Cytopathol. 2019; 128:238–49.

11. Kim M, Park HJ, Min HS, et al. The use of the Bethesda System for Reporting Thyroid Cytopathology in Korea: a nationwide multicenter survey by the Korean Society of Endocrine Pathologists. J Pathol Transl Med. 2017; 51:410–7.

12. Bychkov A, Kakudo K, Hong S. Current practices of thyroid fine-needle aspiration in Asia: a missing voice. J Pathol Transl Med. 2017; 51:517–20.

13. Kakudo K, Higuchi M, Hirokawa M, Satoh S, Jung CK, Bychkov A. Thyroid FNA cytology in Asian practice-Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology. 2017; 28:455–66.

14. Kakudo K, Jung CK, Liu Z, et al. The Asian Thyroid Working Group, from 2017 to 2023. J Pathol Transl Med. 2023; 57:289–304.

15. Jung CK, Hong S, Bychkov A, Kakudo K. The use of fine-needle aspiration (FNA) cytology in patients with thyroid nodules in Asia: a brief overview of studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med. 2017; 51:571–8.

16. Japan Thyroid Association. Guidelines for clinical practice for the management of thyroid nodules in Japan 2013. Tokyo: Nankodo Co., Ltd;2013.
17. Kini SR. Thyroid cytopathology: an atlas and text. Philadelphia: Lippincott Williams and Wilkins;2008.
18. Lloyd R, Osamura R, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. Lyon: IARC Press;2017. 4th.
19. Ha EJ, Na DG, Baek JH. Korean Thyroid Imaging Reporting and Data System: current status, challenges, and future perspectives. Korean J Radiol. 2021; 22:1569–78.

20. Miranda-Filho A, Lortet-Tieulent J, Bray F, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021; 9:225–34.

21. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid cancer incidence trends in the United States: association with changes in professional guideline recommendations. Thyroid. 2020; 30:1132–40.

22. Rana C, Vuong HG, Nguyen TQ, et al. The incidence of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a meta-analysis assessing worldwide impact of the reclassification. Thyroid. 2021; 31:1502–13.

23. Bychkov A, Jung CK, Liu Z, Kakudo K. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol. 2018; 29:276–88.

24. Bychkov A, Hirokawa M, Jung CK, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian Practice. Thyroid. 2017; 27:983–4.

25. Zhu Y, Li Y, Jung CK, et al. Histopathologic assessment of capsular invasion in follicular thyroid neoplasms: an observer variation study. Endocr Pathol. 2020; 31:132–40.

26. Bhattacharyya N. Survival and prognosis in Hurthle cell carcinoma of the thyroid gland. Arch Otolaryngol Head Neck Surg. 2003; 129:207–10.

27. Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer. 2000; 89:202–17.

28. Agarwal S, Bychkov A, Jung CK, et al. The prevalence and surgical outcomes of Hurthle cell lesions in FNAs of the thyroid: a multi-institutional study in 6 Asian countries. Cancer Cytopathol. 2019; 127:181–91.
29. Thompson LD, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol. 2018; 29:242–9.

30. Liu Z, Bychkov A, Jung CK, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int. 2019; 69:202–10.

31. Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016; 26:1535–40.

32. Lee JH, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet. 2014; 384:1848.

33. Kakudo K, Kameyama K, Miyauchi A, Nakamura H. Introducing the reporting system for thyroid fine-needle aspiration cytology according to the new guidelines of the Japan Thyroid Association. Endocr J. 2014; 61:539–52.

34. Kameyama K, Sasaki E, Sugino K, Ito K. The Japanese Thyroid Association Reporting System of thyroid aspiration cytology and experience from a high-volume center, especially in indeterminate category. J Basic Clin Med. 2015; 4:70–4.
35. Satoh S, Yamashita H, Kakudo K. Thyroid Cytology: the Japanese system and experience at Yamashita Thyroid Hospital. J Pathol Transl Med. 2017; 51:548–54.

36. Boon ME, Lowhagen T, Willems JS. Planimetric studies on fine needle aspirates from follicular adenoma and follicular carcinoma of the thyroid. Acta Cytol. 1980; 24:145–8.
37. Crissman JD, Drozdowicz S, Johnson C, Kini SR. Fine needle aspiration diagnosis of hyperplastic and neoplastic follicular nodules of the thyroid: a morphometric study. Anal Quant Cytol Histol. 1991; 13:321–8.
38. Luck JB, Mumaw VR, Frable WJ. Fine needle aspiration biopsy of the thyroid. Differential diagnosis by videoplan image analysis. Acta Cytol. 1982; 26:793–6.
39. Wright RG, Castles H, Mortimer RH. Morphometric analysis of thyroid cell aspirates. J Clin Pathol. 1987; 40:443–5.

40. Gupta S, Savala R, Gupta N, Dey P. Fractal dimension and chromatin textural analysis to differentiate follicular carcinoma and adenoma on fine needle aspiration cytology. Cytopathology. 2020; 31:491–3.

41. Savala R, Dey P, Gupta N. Artificial neural network model to distinguish follicular adenoma from follicular carcinoma on fine needle aspiration of thyroid. Diagn Cytopathol. 2018; 46:244–9.

42. Han K, Ha HJ, Kong JS, et al. Cytological features that differentiate follicular neoplasm from mimicking lesions. J Pathol Transl Med. 2018; 52:110–20.

43. Cervino JM, Paseyro P, Grosso OF, Maggiolo J. The cytological examination of the thyroid gland and its anatomo-clinical correlations. An Fac Med Univ Repub Montev Urug. 1962; 47:128–43.
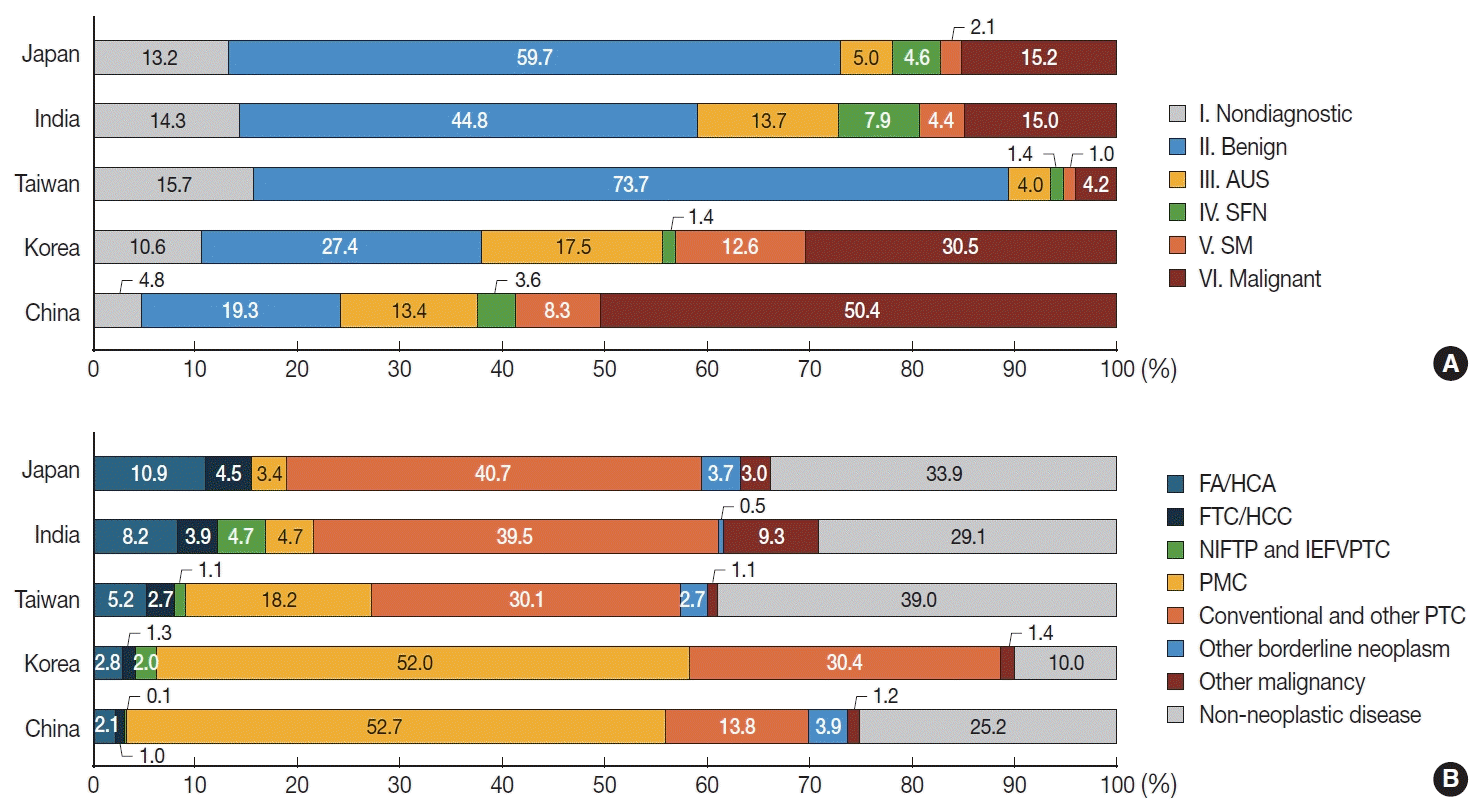
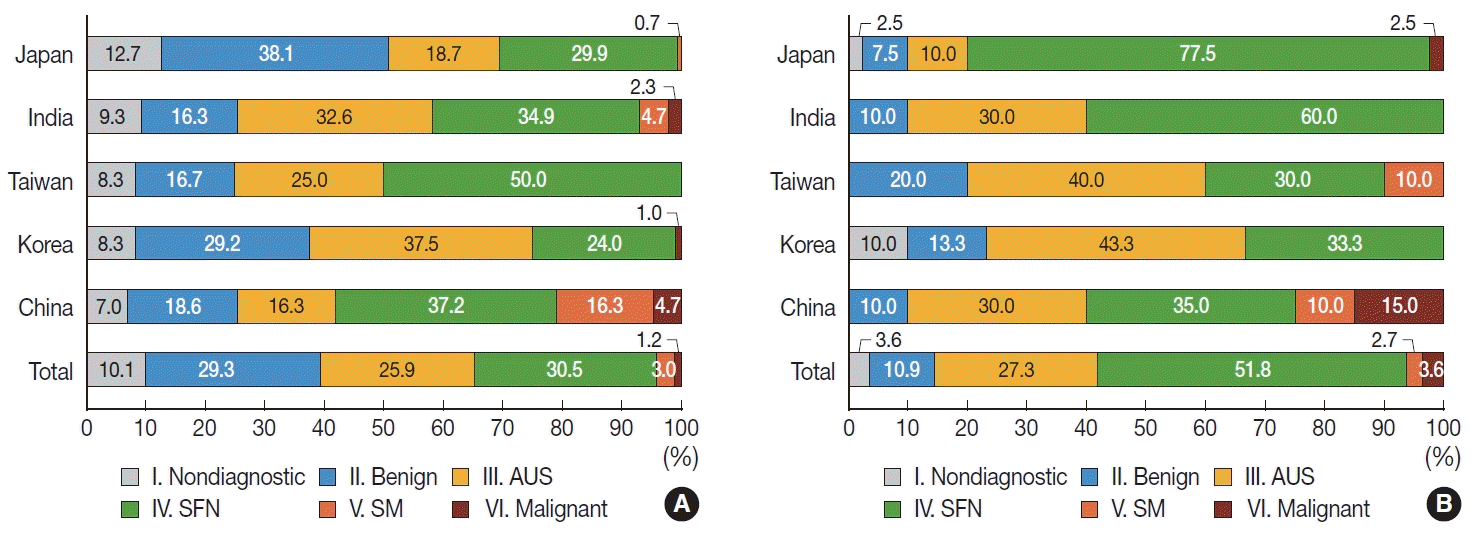
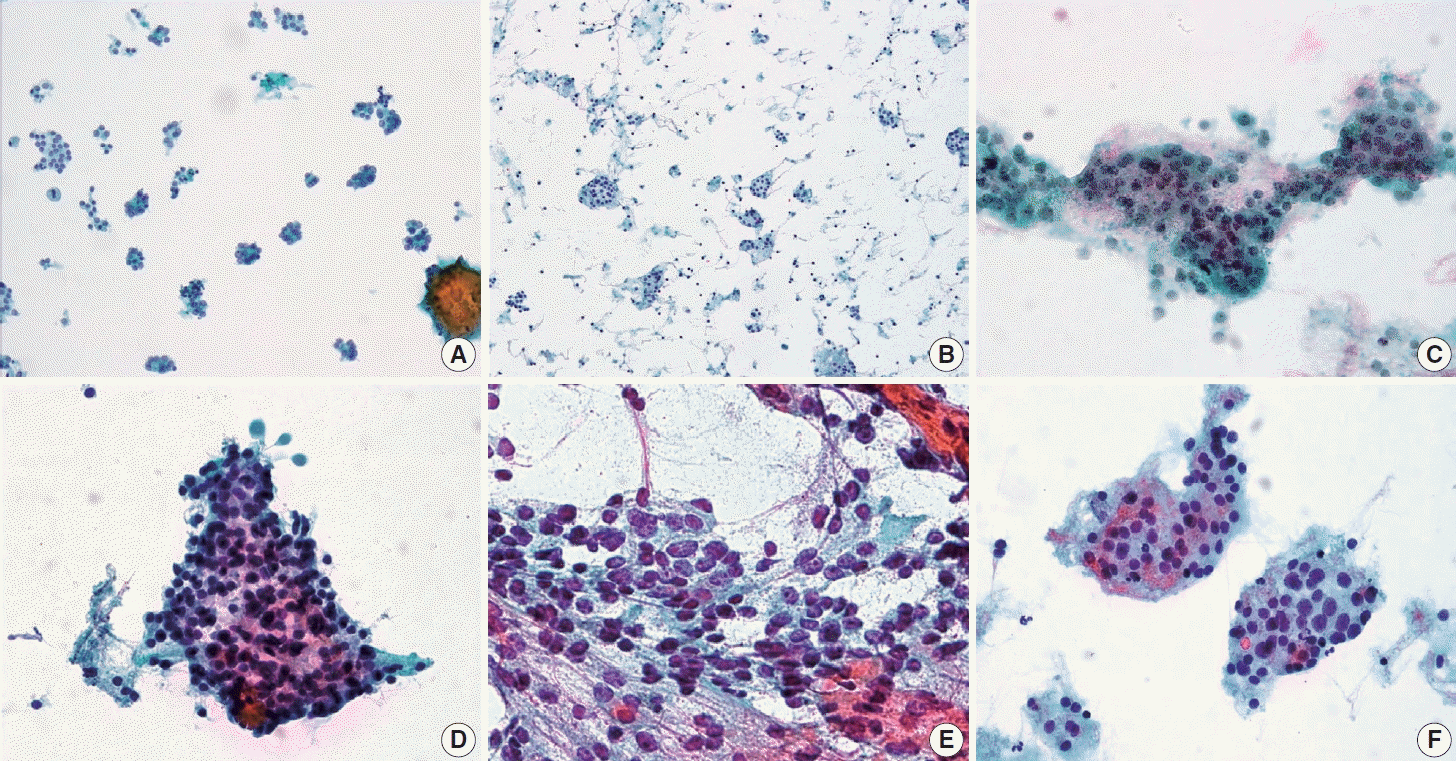




 PDF
PDF Citation
Citation Print
Print



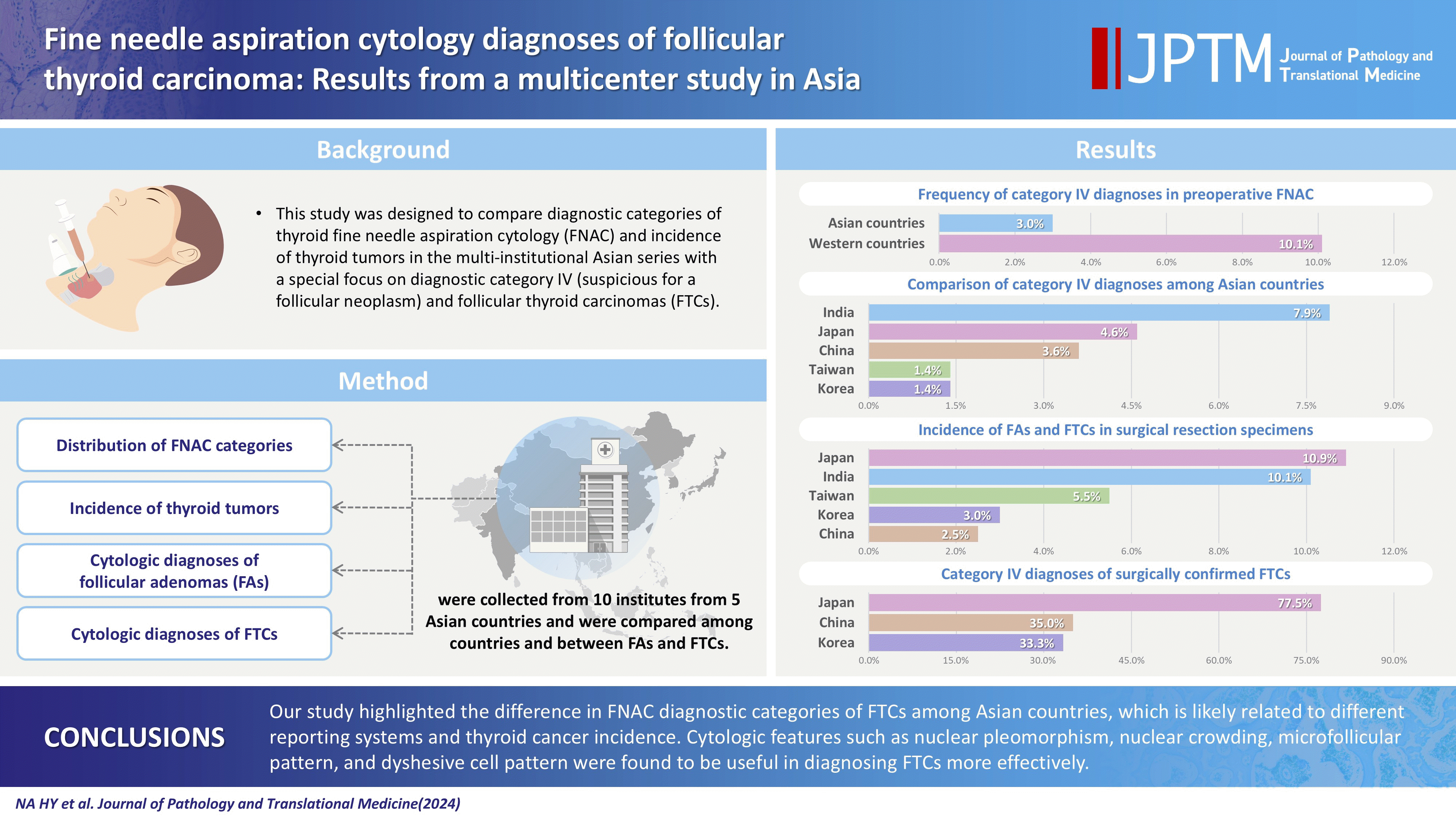
 XML Download
XML Download