Abstract
Cervical cancer screening during pregnancy presents unique challenges for cytologic interpretation. This review focuses on pregnancy-associated cytomorphological changes and their impact on diagnosis of cervical intraepithelial neoplasia (CIN) and cervical cancer. Pregnancy-induced alterations include navicular cells, hyperplastic endocervical cells, immature metaplastic cells, and occasional decidual cells or trophoblasts. These changes can mimic abnormalities such as koilocytosis, adenocarcinoma in situ, and high-grade squamous intraepithelial lesions, potentially leading to misdiagnosis. Careful attention to nuclear features and awareness of pregnancy-related changes are crucial for correct interpretation. The natural history of CIN during pregnancy shows higher regression rates, particularly for CIN 2, with minimal risk of progression. Management of abnormal cytology follows modified risk-based guidelines to avoid invasive procedures, with treatment typically deferred until postpartum. The findings reported in this review emphasize the importance of considering pregnancy status in cytological interpretation, highlight potential problems, and provide guidance on differentiating benign pregnancy-related changes from true abnormalities. Understanding these nuances is essential for accurate diagnosis and proper management of cervical abnormalities in pregnant women.
Cervical cancer is the most common gynecological malignancy in pregnant women, occurring in approximately 1.6–11.1 cases in 100,000 pregnancies, and 3% of cervical cancer cases are diagnosed in pregnancy [1-3]. The incidence of cervical intraepithelial neoplasia (CIN), the direct precursor of cervical cancer, usually peaks during child-bearing age, with significantly higher incidence of approximately 133 cases in 100,000 pregnancies [4]. Because cytologic screening of cervical cancer has become an essential part of standard antenatal care, encountering cervical cytology specimens from pregnant women is a common daily practice for pathologists [5]. However, the interpretation and diagnosis of cervical cytology during pregnancy can be challenging due to specific changes associated with pregnancy. In this review, we aim to review some interesting features of cervical cancer and CIN during pregnancy with emphasis on the cytologic findings and potential issues in interpretation.
Although cervical cancer remains a very rare condition, the peak incidence of CIN, the direct precursor of cervical cancer, usually occurs in child-bearing age. The increasing trend of delayed childbearing has led to a significant increase in the incidence of cervical cancer and CIN during pregnancy in recent decades [6,7]. Consequently, Papanicolaou (Pap) screening has been established as a standard procedure in routine antenatal care [5]. Notably, pregnancy can provide an opportunity to screen for cervical cancer in women who otherwise would not be tested. Abnormal Pap results are reported in approximately 3.3%–5% of pregnant women, comparable with non-pregnant women [8,9].
Human papillomavirus (HPV) DNA testing has recently been implemented in cervical cancer screening as either a standalone screening modality or as a co-test with cytology [10-12]. HPV DNA testing has also been utilized for cervical cancer screening in pregnant women with satisfactory results [13]. However, HPV DNA testing is considered less specific, particularly in young women, due to a higher prevalence of HPV infection in women under 30 years of age. In a recent literature review over a 10-year period, Pap testing was an important first modality for cervical cancer screening in pregnant women [14].
Pregnancy incurs profound physiological changes in the cervical and endocervical mucosa causing various morphologic alterations on cytology. Knowing the pregnancy status of a patient is pertinent to avoid misinterpretation. In pregnancy, the squamous epithelium of cervical mucosa reamins less mature due to lack of estrogen-driven differentiation. The cervix also shows transformation zone eversion, increased vascularity, endocervical glandular hyperplasia, and increased mucus production. On rare occasions, a small number of degraded cells from decidua or trophoblasts may be shed and released. All these changes may cause cytologic alterations and can cause potential issues in interpretation.
Navicular cells are intermediate squamous epithelial cells with glycogen-rich cytoplasm and a boat-like (navicular) shape (Fig. 1A) [15]. These cells may be present in the normal secretory phase, but the number increases as gestation proceeds. These cells sometimes can be misinterpreted as koilocytes. The differential aspect is the presence of pale yellowish cytoplasm with a vague outline instead of the clear halo around the nuclei with defined border corresponding to the prefix “koilo” (meaning empty) in koilocytes. The nuclei of navicular cells are small and typical, while those of koilocytes are enlarged, raisinoid, hyperchromatic, and sometimes multinucleated (Fig. 1B).
Endocervical glandular hyperplasia often results in an abundance of glandular epithelium on sampling, with increased mucin. These glandular cells are sometimes confused with adenocarcinoma in situ (AIS) and may be diagnosed as atypical glandular cells (AGCs). The differential diagnosis is based on the regular honeycomb appearance of the glandular cluster with low nuclear-cytoplasmic (N/C) ratio. The cytoplasm of these glandular clusters is always full of clear mucin (Fig. 2A). Conversely, AIS cells usually have less mucinous cytoplasm and stratified nuclei and are pencil-like and hyperchromatic. The cells often show typical feathering at the outer edges of the clusters (Fig. 2B).
Ectropion, eversion of the uterine cervix, can result in exposure of the transformation zone, which in combination with the decreased maturation, results in numerous immature metaplastic cells in the cytologic specimen. These cells can be confused with ASC-H (atypical squamous cells - cannot exclude high grade squamous intraepithelial lesion) or high-grade squamous intraepithelial lesion (HSIL), causing difficulty in diagnosis (Fig. 3A). Differentiation between ASC-H and HSIL is mainly based on scrutinization of the nuclear features. ASC-H and HSIL have enlarged hyperchromatic nuclei with coarse chromatin. The nuclear membrane is often irregular and thickened in ASC-H and HSIL (Fig. 3B).
The Arias-Stella reaction is a specific cellular change in gestation, usually observed in endometrial and sometimes endocervical glandular cells. Affected cells show markedly pleomorphic, atypical, hyperchromatic nuclei with occasional prominent nucleoli, sometimes having a hobnail appearance. The cells have abundant clear or vacuolated cytoplasm. On cytologic samples, detached glandular cells with Arias-Stella reaction can easily be misdiagnosed as AGC or adenocarcinoma, especially if the pathologist is not informed of the pregnancy status. Careful attention to the cytological details including low N/C ratio, ample lacy cytoplasm, smooth or indistinct nuclear outline, and dull opaque rather than coarse chromatin pattern aids in the differentiation of this specific benign condition from malignancy (Fig. 4).
Degenerated decidual cells or trophoblasts may be released and sampled in cytologic specimens, although infrequently. These cells may be considered as abnormal squamous cells due to their glossy ample cytoplasm and slightly large hyperchromatic nuclei and misdiagnosed as ASC or ASC-H [16-18]. Decidual cells usually do not demonstrate the high N/C ratio and coarse chromatin pattern of HSIL, although they sometimes show prominent nucleoli (Fig. 5).
Although the natural course of CIN in pregnant women does not differ significantly from non-pregnant women, CIN during pregnancy has a few notable features. Progression to invasion is extremely rare in CIN during pregnancy. Most cases remain stable and many even regress. The overall regression rate for CIN during pregnancy is estimated to be as high as 76% for low-grade squamous intraepithelial lesions (LSILs) and up to 59% for HSILs [19-23]. Although some heterogeneous results have been reported, the regression rate of CIN during pregnancy is generally accepted to be much higher than for non-pregnant women [21].
In general, irrespective of pregnancy status, more than 80% of HPV infections resolve spontaneously, with approximately 10%–20% of HPV-infected women developing CIN [24]. In LSIL/CIN 1, approximately 57% of cases regress, 32% persist, and approximately 12% progress to high-grade lesions [25]. For high-grade lesions, such as CIN 3, the regression rates decrease significantly, with almost half persisting and more than 12% of the cases progressing to invasive carcinoma [25].
For LSIL/CIN 1 occurring in pregnancy, regression rates are higher, in the range of 63%–76%, and progression rates are low (6%–8%). The overall regression rate for HSIL (CIN 2/CIN 3) during pregnancy ranges from 29%–59% [19-23]. In a previous meta-analysis, the pooled regression rate for HSIL during pregnancy was 40% (95% confidence interval [CI], 35% to 45%) [21]. CIN 2 had a significantly higher regression rate compared with CIN 3 (59%–88% vs. 21%–29%) [19-23]. Most regression tends to occur within the first 2 years postpartum. In a previous study, approximately 68%–70% of regressions (for both CIN 2 and CIN 3) were observed within the first 2 years after diagnosis [22]. The progression rate to invasive cancer is very low, typically around 1% (95% CI, 0% to 2%) [21], which becomes the basis for a more conservative approach in management of CIN during pregnancy.
The mechanism of frequent CIN regression during pregnancy is not yet completely understood. Some proposed hypotheses include pregnancy-induced immunologic alteration, inflammatory process, and cervical repair related to delivery. Cervical trauma during vaginal delivery and subsequent repair were suggested to contribute to regression and was supported in a few studies in which regression was more frequent in vaginal delivery [19,26,27]. However, other studies have shown that regression rates do not differ significantly between vaginal deliveries and cesarean sections, challenging this hypothesis [28-30]. In a recent meta-analysis, delivery mode did not affect regression [31]. The hypothesis of cervical repair and the impact of delivery mode on CIN regression should be further investigated.
The observation that CIN 2 regresses more frequently than CIN 3 is consistent across multiple studies [21] and reasonable considering that CIN 2 more commonly regresses than CIN 3 in non-pregnant cases.
High-risk HPV infection, particularly HPV 16/18, and HPV E6/E7 mRNA expression are reported to correlate with disease persistence and/or progression [30,32,33].
There are no other prominent cytological or histological features known to predict regression. Stromal inflammation was reportedly associated with regression and can be associated with an immunologic reaction that may have a role in regression [34]. However, measuring stromal inflammation accurately is difficult and can be interpreted as non-specific. Morphological differences do not exist between regressed and persisted/progressed cases (Figs. 6, 7) [34].
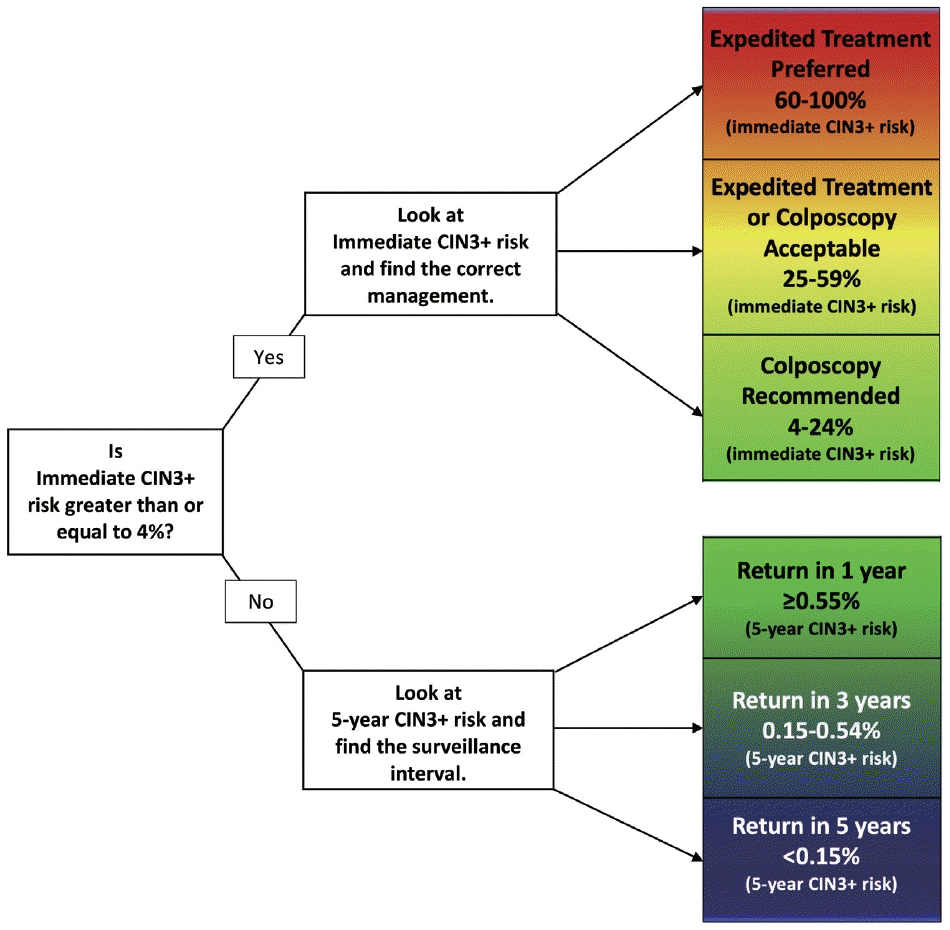
Pregnancy does not alter the management approach of abnormal screening test results. According to the 2019 American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines, the management algorithm is divided based on immediate risk of CIN 3+ (CIN 3 or worse) > 4% (Fig. 8) [35,36]. This risk is calculated using a complex tabulation including patient age, interval since last screening, and current and prior test results. HSIL, ASC-H, and AGC on cytology as well as HPV-16 and HPV-18 on HPV test always require colposcopy and/or biopsy [37]. Although the risk threshold of 4% for colposcopy referral are not altered during pregnancy, endocervical curettage, endometrial biopsy, and expedited treatment are not acceptable [35]. In pregnant women, all diagnostic approaches should be directed to exclude invasive cervical carcinoma [38]. During pregnancy, patients with histologically confirmed CIN 2 or CIN 3 are recommended to be under active surveillance with repeat colposcopy every 12 to 24 weeks. However, it is acceptable to defer colposcopy until postpartum [35]. Treatment of histologic HSIL is not recommended. Diagnostic excisional procedure or repeat biopsy should be postponed until after delivery unless invasive carcinoma is suspected [35].
Cervical cytology has become an integral part of routine antenatal care and remains a crucial step in screening for cervical cancer in women. Pathologists should consider patient age and pregnancy status when examining cytology samples. Understanding the cytologic features of pregnancy-related changes and potential issues is important to avoid misdiagnosis. Generally, caution is advises to not overdiagnose cytology samples from pregnant women because HSIL frequently regresses and may not be detected during postpartum follow-up. However, unequivocal HSIL should be classified as HSIL regardless of the patient’s pregnancy status.
Notes
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
1. Nguyen C, Montz FJ, Bristow RE. Management of stage I cervical cancer in pregnancy. Obstet Gynecol Surv. 2000; 55:633–43.

2. Norstrom A, Jansson I, Andersson H. Carcinoma of the uterine cervix in pregnancy: a study of the incidence and treatment in the western region of Sweden 1973 to 1992. Acta Obstet Gynecol Scand. 1997; 76:583–9.

3. Pentheroudakis G, Pavlidis N. Cancer and pregnancy: poena magna, not anymore. Eur J Cancer. 2006; 42:126–40.

4. Al-Halal H, Kezouh A, Abenhaim HA. Incidence and obstetrical outcomes of cervical intraepithelial neoplasia and cervical cancer in pregnancy: a population-based study on 8.8 million births. Arch Gynecol Obstet. 2013; 287:245–50.

6. Eibye S, Kjaer SK, Mellemkjaer L. Incidence of pregnancy-associated cancer in Denmark, 1977-2006. Obstet Gynecol. 2013; 122:608–17.

7. Eibye S, Kruger Kjaer S, Nielsen TS, Mellemkjaer L. Mortality among women with cervical cancer during or shortly after a pregnancy in Denmark 1968 to 2006. Int J Gynecol Cancer. 2016; 26:951–8.

8. Yang KY. Abnormal pap smear and cervical cancer in pregnancy. Clin Obstet Gynecol. 2012; 55:838–48.

9. Suzuki S, Hayata E, Hoshi SI, et al. Current status of cervical cytology during pregnancy in Japan. PLoS One. 2021; 16:e0245282.

10. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015; 125:330–7.
11. Watson M, Benard V, King J, Crawford A, Saraiya M. National assessment of HPV and Pap tests: changes in cervical cancer screening, National Health Interview Survey. Prev Med. 2017; 100:243–7.

12. Xhaja A, Ahr A, Zeiser I, Ikenberg H. Two years of cytology and HPV co-testing in Germany: initial experience. Geburtshilfe Frauenheilkd. 2022; 82:1378–86.

13. Gu L, Hu Y, Wei Y, et al. Optimising cervical cancer screening during pregnancy: a study of liquid-based cytology and HPV DNA cotest. Epidemiol Infect. 2024; 152:e25.

14. Zagorianakou N, Mitrogiannis I, Konis K, Makrydimas S, Mitrogiannis L, Makrydimas G. The HPV-DNA test in pregnancy: a review of the literature. Cureus. 2023; 15:e38619.

16. Cibas ES, Ducatman BS. Cytology: diagnostic principles and clinical correlates. Philadelphia: Elsevier;2021. 5th.
17. Origoni M, Salvatore S, Perino A, Cucinella G, Candiani M. Cervical Intraepithelial Neoplasia (CIN) in pregnancy: the state of the art. Eur Rev Med Pharmacol Sci. 2014; 18:851–60.
18. Grimm D, Lang I, Prieske K, et al. Course of cervical intraepithelial neoplasia diagnosed during pregnancy. Arch Gynecol Obstet. 2020; 301:1503–12.

19. Stuebs FA, Mergel F, Koch MC, et al. Cervical intraepithelial neoplasia grade 3: development during pregnancy and postpartum. Arch Gynecol Obstet. 2023; 307:1567–72.

20. Dasgupta S. The fate of cervical dysplastic lesions during pregnancy and the impact of the delivery mode: a review. Cureus. 2023; 15:e42100.

21. Chen C, Xu Y, Huang W, Du Y, Hu C. Natural history of histologically confirmed high-grade cervical intraepithelial neoplasia during pregnancy: meta-analysis. BMJ Open. 2021; 11:e048055.

22. Ehret A, Bark VN, Mondal A, Fehm TN, Hampl M. Regression rate of high-grade cervical intraepithelial lesions in women younger than 25 years. Arch Gynecol Obstet. 2023; 307:981–90.

23. Han B, Yuan M, Gong Y, et al. The clinical course of untreated CIN2 (HPV16/18+) under active monitoring: a protocol of systematic reviews and meta-analysis. Medicine (Baltimore). 2023; 102:e32855.

24. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998; 338:423–8.

25. Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993; 12:186–92.
26. Ahdoot D, Van Nostrand KM, Nguyen NJ, et al. The effect of route of delivery on regression of abnormal cervical cytologic findings in the postpartum period. Am J Obstet Gynecol. 1998; 178:1116–20.

27. Chung SM, Son GH, Nam EJ, et al. Mode of delivery influences the regression of abnormal cervical cytology. Gynecol Obstet Invest. 2011; 72:234–8.

28. Coppola A, Sorosky J, Casper R, Anderson B, Buller RE. The clinical course of cervical carcinoma in situ diagnosed during pregnancy. Gynecol Oncol. 1997; 67:162–5.
29. Bracic T, Reich O, Taumberger N, Tamussino K, Trutnovsky G. Does mode of delivery impact the course of cervical dysplasia in pregnancy? A review of 219 cases. Eur J Obstet Gynecol Reprod Biol. 2022; 274:13–8.

30. Cubo-Abert M, Centeno-Mediavilla C, Franco-Zabala P, et al. Risk factors for progression or persistence of squamous intraepithelial lesions diagnosed during pregnancy. J Low Genit Tract Dis. 2012; 16:34–8.

31. Douligeris A, Pergialiotis V, Pappa K, et al. The effect of the delivery mode on the evolution of cervical intraepithelial lesions during pregnancy: a meta-analysis. J Gynecol Obstet Hum Reprod. 2022; 51:102462.

32. Frega A, Verrone A, Manzara F, et al. Expression of E6/E7 HPV-DNA, HPV-mRNA and colposcopic features in management of CIN2/3 during pregnancy. Eur Rev Med Pharmacol Sci. 2016; 20:4236–42.
33. Hong DK, Kim SA, Lim KT, Lee KH, Kim TJ, So KA. Clinical outcome of high-grade cervical intraepithelial neoplasia during pregnancy: a 10-year experience. Eur J Obstet Gynecol Reprod Biol. 2019; 236:173–6.

34. Pongsuvareeyakul T, Eaton S, Quddus MR, Sung CJ, Singh K. Comparison of cervical HSIL outcome between pregnant and non-pregnant women. Ann Clin Lab Sci. 2022; 52:544–55.
35. Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP Risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020; 24:102–31.

36. Nayar R, Chhieng DC, Crothers B, et al. Moving forward-the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020; 9:291–303.

Fig. 1.
(A) Navicular cells (arrows) are intermediate squamous epithelial cells with glycogen-rich cytoplasm and a boat-like (navicular) shape. (B) Navicular cells should be differentiated from koilocytes (arrows) of low-grade squamous intraepithelial lesion (ThinPrep, Papanicolaou stain).
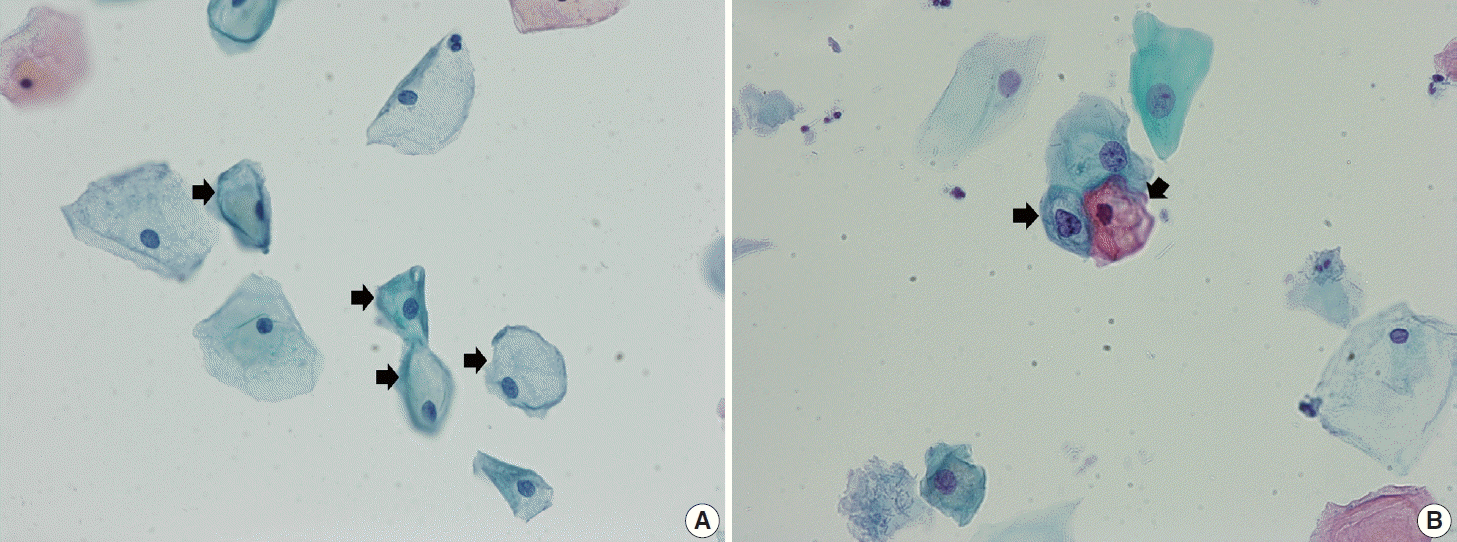
Fig. 2.
(A) Endocervical fragments in pregnancy in a regular honeycomb structure showing clear cytoplasm filled with mucin. (B) Glandular fragments of adenocarcinoma in situ showing stratified pencil-like, hyperchromatic nuclei with feathering at the edge of the cluster (conventional smear, Papanicolaou stain).
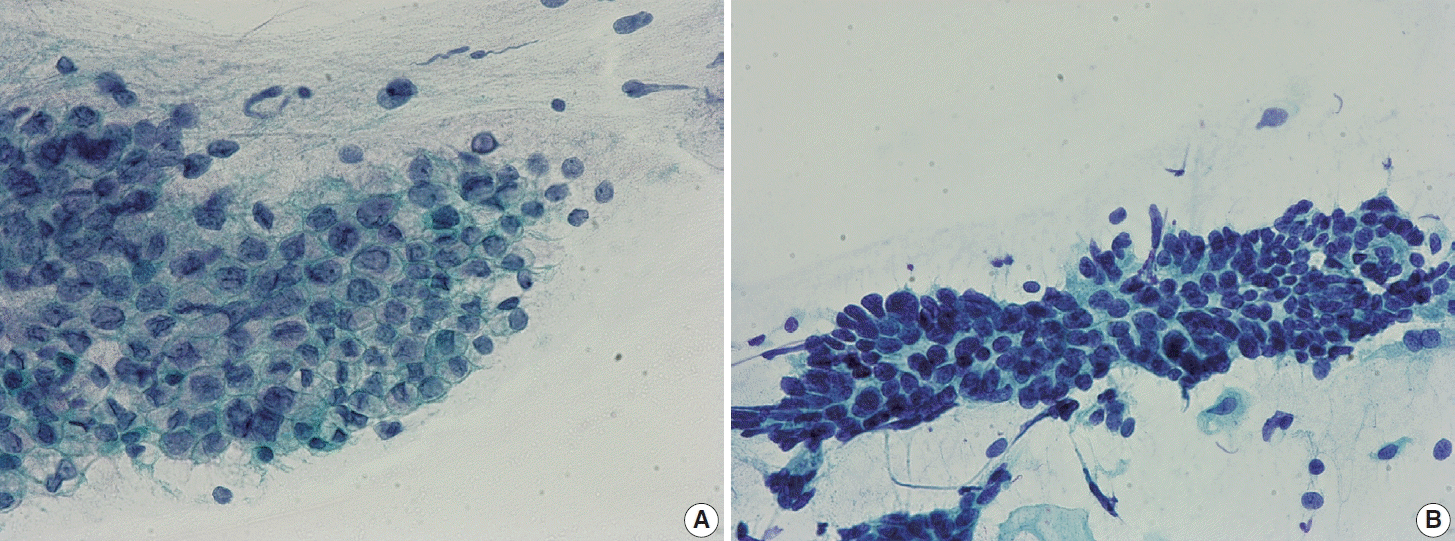
Fig. 3.
(A) Immature metaplastic cells in pregnancy (arrows) showing ample glossy cytoplasm with slightly increased nuclear-cytoplasmic (N/C) ratio. (B) Atypical squamous cells - cannot exclude high grade squamous intraepithelial lesion cells with higher N/C ratio and hyperchromatic nuclei with irregular nuclear membrane and coarse chromatin (ThinPrep, Papanicolaou stain).
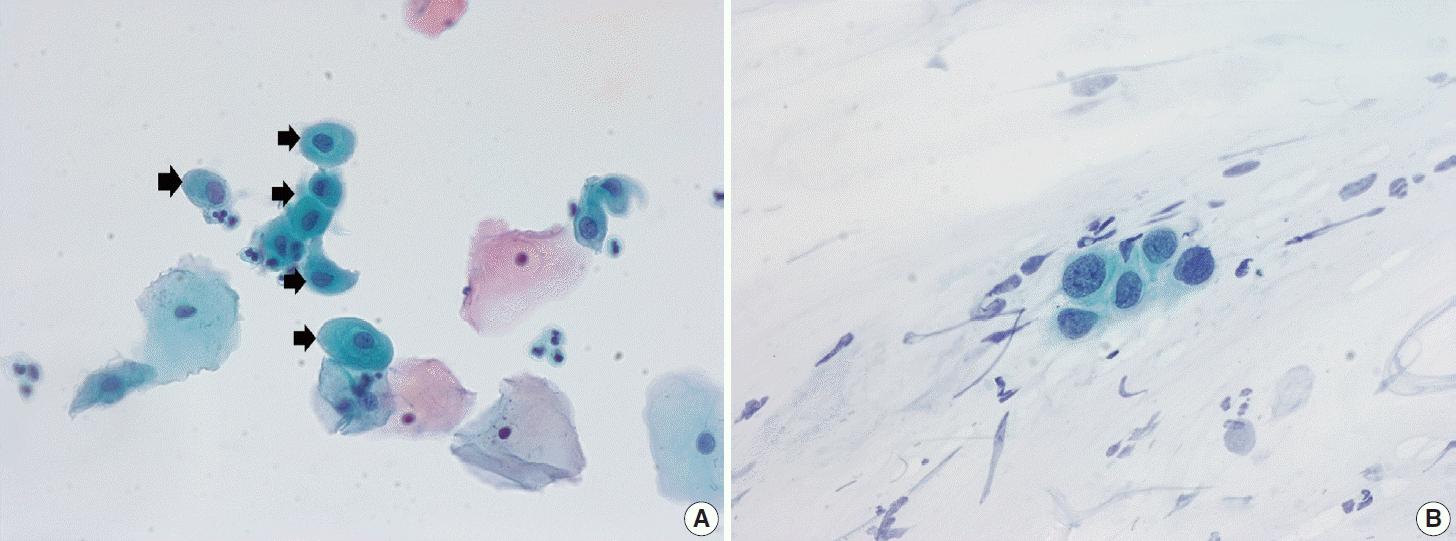
Fig. 4.
(A) Cluster of glandular cells showing the Arias-Stella reaction. The cells have low nuclear-cytoplasmic (N/C) ratio and ample lacy cytoplasm. The nuclei are dull and opaque with fuzzy outlines. (B) A few scattered glandular cells in the Arias-Stella reaction (arrows). Although the nuclei are enlarged with prominent nucleoli, the N/C ratio remains low, and the chromatin is smudged rather than coarse (ThinPrep, Papanicolaou stain).
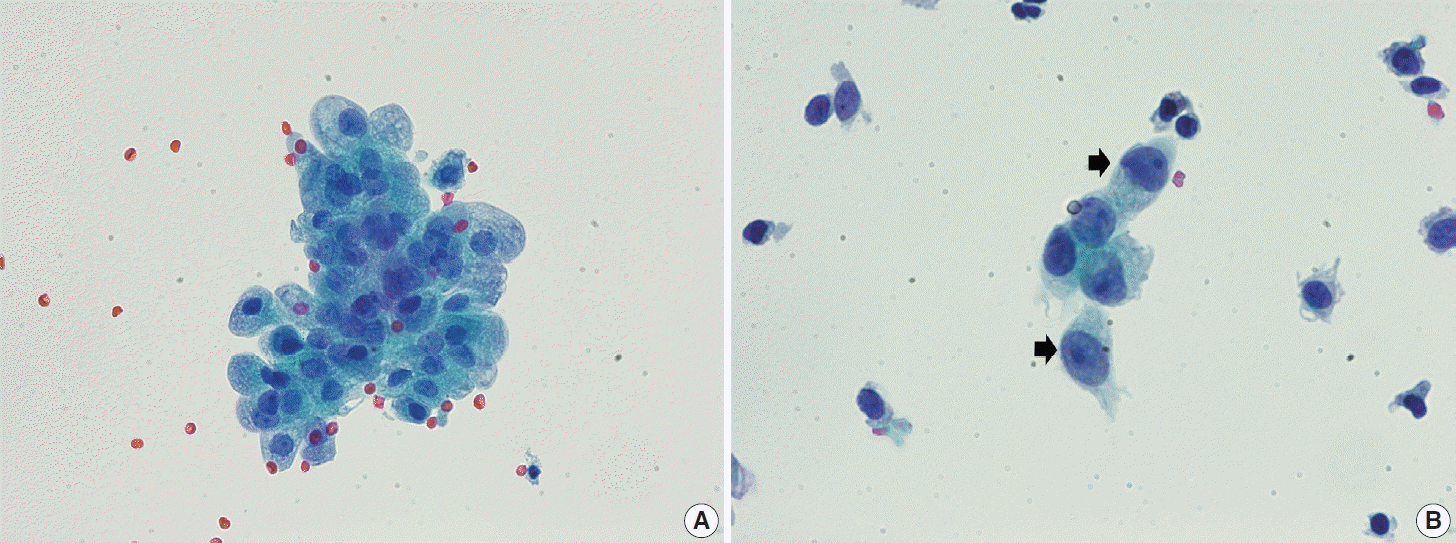
Fig. 5.
(A) Decidual cells in pregnancy (arrowheads). These cells usually have ample thick cytoplasm. The nuclear-cytoplasmic ratio is low with smooth round nuclei. (B) Trophoblastic villi (arrow) and single or multinucleated trophoblasts (arrowheads) in pregnancy. A few decidual cells is observed in the background (empty arrow) (ThinPrep, Papanicolaou stain).
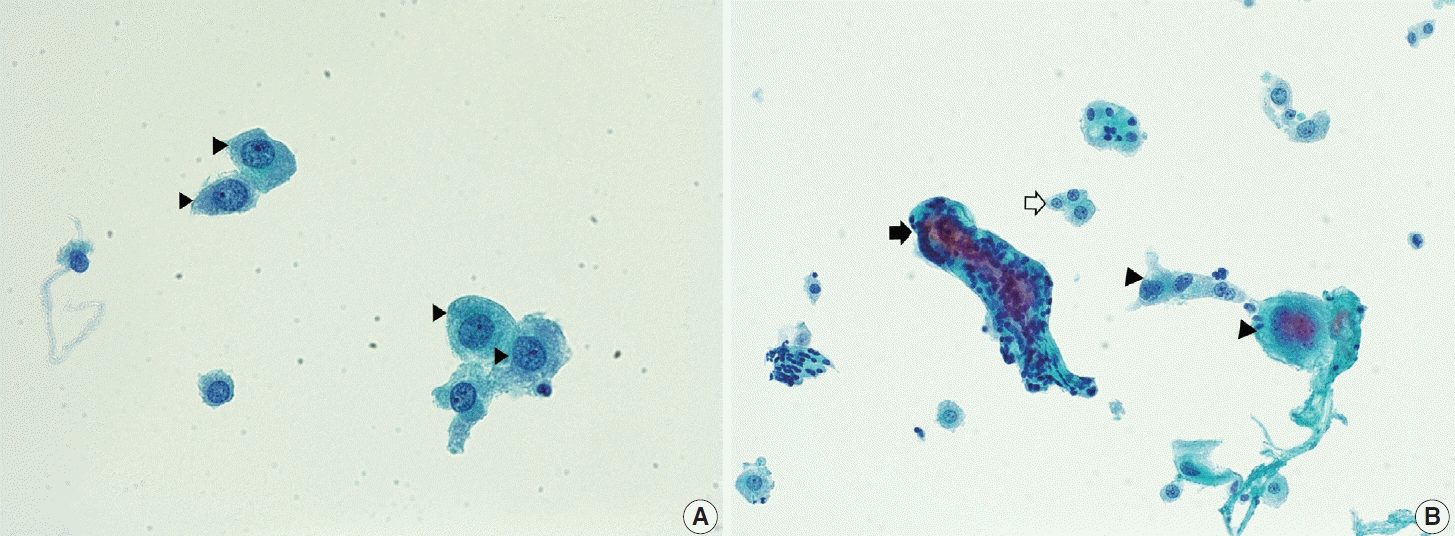
Fig. 6.
(A) A case of persisting high-grade squamous intraepithelial lesion (HSIL). The cellular cluster shows enlarged hyperchromatic nuclei, irregular nuclear membrane, and rather coarse chromatin discernible at the periphery of the cluster. (B) Another case of persisting HSIL. A sheet-like cluster of cells with high nuclear-cytoplasmic ratio and hyperchromatic nuclei (ThinPrep, Papanicolaou stain).
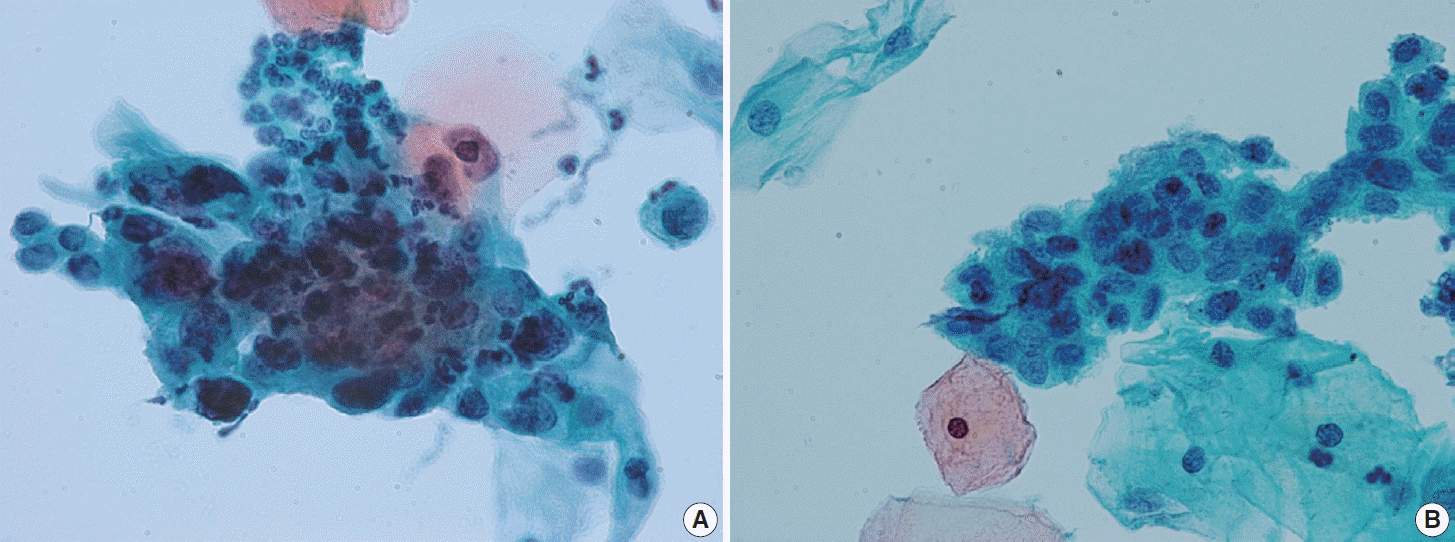
Fig. 7.
(A) A case of regressed high-grade squamous intraepithelial lesion (HSIL). HSIL cluster showing enlarged hyperchromatic nuclei. Cytological differences from persisting HSIL cases are not obvious. (B) Another case of regressed HSIL. The loosely cohesive HSIL cells show high nuclear-cytoplasmic ratio and hyperchromatic nuclei with irregular nuclear membrane and coarse chromatin. Specific differences were not observed in cytomorphological features between persisting and regressed cases of HSIL (ThinPrep, Papanicolaou stain).
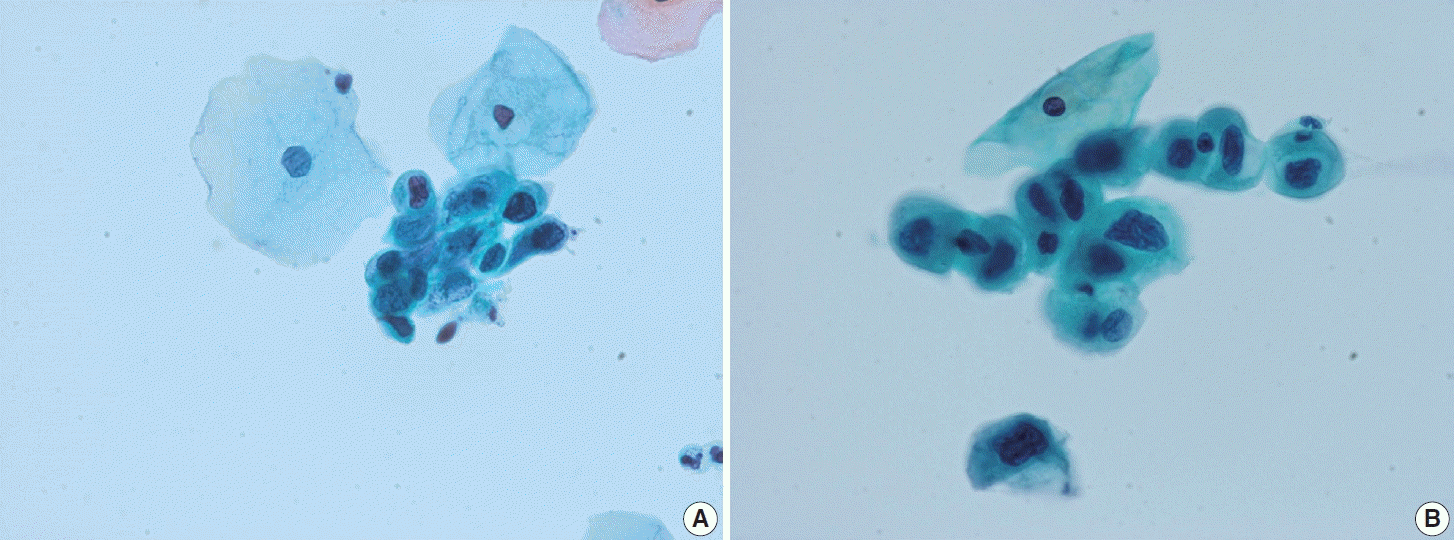
Fig. 8.
2019 American Society for Colposcopy and Cervical Pathology (ASCCP) management guideline. The figure shows how the patient is managed. If the calculated risk of immediate CIN3+ is ≥ 4%, immediate management via colposcopy or treatment is indicated. Reprinted from Nayar et al. J Am Soc Cytopathol 2020; 9: 291-303 [36], with permisison of Elsevier.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download