Abstract
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, characterized by a range of subtypes that differ in their cytologic features, clinical behavior, and prognosis. Accurate cytologic evaluation of PTC using fine-needle aspiration is essential but can be challenging due to the morphologic diversity among subtypes. This review focuses on the distinct cytologic characteristics of various PTC subtypes, including the classic type, follicular variant, tall cell, columnar cell, hobnail, diffuse sclerosing, Warthin-like, solid/trabecular, and oncocytic PTCs. Each subtype demonstrates unique nuclear features, architectural patterns, and background elements essential for diagnosis and differentiation from other thyroid lesions. Recognizing these distinct cytologic patterns is essential for identifying aggressive subtypes like tall cell, hobnail, and columnar cell PTCs, which have a higher risk of recurrence, metastasis, and poorer clinical outcomes. Additionally, rare subtypes such as diffuse sclerosing and Warthin-like PTCs present unique cytologic profiles that must be carefully interpreted to avoid diagnostic errors. The review also highlights the cytologic indicators of lymph node metastasis and high-grade features, such as differentiated high-grade thyroid carcinoma. The integration of molecular testing can further refine subtype diagnosis by identifying specific genetic mutations. A thorough understanding of these subtype-specific cytologic features and molecular profiles is vital for accurate diagnosis, risk stratification, and personalized management of PTC patients. Future improvements in diagnostic techniques and standardization are needed to enhance cytologic evaluation and clinical decision-making in thyroid cancer.
In recent decades, there has been a significant increase in the incidence of thyroid cancer, primarily due to improved detection methods and greater public awareness [1,2]. Thyroid cancer is the fifth most prevalent malignancy among females and the seventh most common among both sexes worldwide [1]. Papillary thyroid carcinoma (PTC) represents the most prevalent form of thyroid malignancy, accounting for approximately 90% of all thyroid cancer cases. Characterized as a well-differentiated neoplasm, PTC originates from the follicular cells of the thyroid gland and is known for its relatively favorable prognosis and high long-term survival rates [3]. Despite its generally indolent nature, certain aggressive subtypes of PTC are associated with a higher propensity for lymph node metastasis, tumor recurrence, and patient mortality, necessitating careful clinical management and surveillance [4-7].
The cytologic evaluation of thyroid nodules is pivotal in the initial assessment and diagnosis of PTC [8]. Fine-needle aspiration (FNA) cytology stands as the primary diagnostic modality, offering a minimally invasive method for obtaining cellular material from thyroid nodules for examination. The Bethesda System for Reporting Thyroid Cytopathology provides a standardized framework for interpreting FNA results, aiding in the stratification of malignancy risk and guiding clinical management [9,10]. The Korean Thyroid Association guidelines have also adopted the Bethesda System as a standard method for diagnosing thyroid nodules, emphasizing its utility in categorizing cytological findings and informing appropriate clinical decisions in the management of thyroid nodules [11].
While subtyping PTC in surgical specimens for histological assessment is relatively straightforward, subtyping PTC based on cytology alone presents significant challenges and limitations. This includes sample adequacy, the subtlety of distinguishing features, and the lack of pathologists’ experiences with rare subtypes, often leading to underreporting and misclassification [12]. Advances in molecular testing, such as next-generation sequencing, have enhanced the diagnostic precision of FNA cytology by identifying specific genetic mutations associated with PTC subtypes [13,14]. Despite these advancements, the limitations of FNA in reliably subtyping PTC highlight the need for continued improvement in diagnostic techniques and protocols. While the Bethesda System does not mandate the identification of specific PTC subtypes during FNA, it allows for the possibility of favoring or suggesting more prevalent subtypes, such as tall cell PTC, when characteristic features are observed [15].
This review article aims to provide a comprehensive overview of PTC subtypes, focusing on their cytological hallmarks and differential diagnosis. Additionally, we provide an overview of cytological features associated with lymph node metastasis and other aggressive indicators of PTC in liquid-based specimens that can be used to evaluate and stratify the risk for tumor aggressiveness. We also give a brief overview of high-grade neoplasms such as differentiated high-grade thyroid carcinoma (DHGTC) and the differentiating cytologic features that set DHGTC apart from PTC.
Thyroid neoplasms constitute a heterogeneous group of tumors originating from the thyroid gland, exhibiting a spectrum of malignancy and clinical behavior. Most thyroid neoplasms arise from follicular epithelial cells and parafollicular cells and are classified under the guidelines provided by the World Health Organization (WHO) [16-18]. The most prevalent types arising from follicular cells are differentiated thyroid carcinomas (DTC), including PTC and follicular thyroid carcinoma (FTC), which generally have a favorable prognosis and respond effectively to treatments such as thyroid hormone suppression and radioiodine therapy [19]. This group of neoplasms demonstrates a spectrum of molecular alterations, predominantly characterized by mutations in the BRAF and RAS genes as primary driver mutations, which critically delineate distinct tumor subtypes with varied clinical behaviors. In contrast, aggressive thyroid neoplasms, such as poorly differentiated thyroid carcinoma (PDTC) and anaplastic thyroid carcinoma (ATC), exhibit poor behavior and adverse outcomes. Distinct molecular alterations play a significant role in the development of these entities. A stepwise progression from DTC is believed to occur due to additional mutations, such as TERT promoter and TP53 mutations [20].
As the most common type of thyroid carcinoma, the diagnosis of classic PTC is characterized by distinctive macroscopic and microscopic features. Gross examination typically reveals a tumor with a solid and papillary architecture, which can present as a well-demarcated or ill-defined mass. Microscopically, PTC exhibits an infiltrative border with papillary, follicular, and solid patterns. The pathognomonic nuclear features crucial for diagnosing PTC include nuclear enlargement, crowding and overlapping, elongation, irregular nuclear contours, nuclear grooves, nuclear pseudoinclusions, and chromatin clearing. Additional findings such as tumor fibrosis and psammoma bodies are frequently observed [21].
Several findings in PTC can influence its clinical behavior. PTC often exhibits multicentric foci in one or both thyroid lobes, lymphatic invasion, and extrathyroidal extension [21]. Vascular invasion is less commonly observed except in the follicular subtype of PTC. Lymph node metastases are frequently seen in PTC, although there is a lower propensity for distant metastasis. The WHO classification delineates several subtypes of PTC based on microscopic appearance and distinct prognostic characteristics. Each subtype presents unique histological and cytological features that can influence the clinical course and prognosis of the disease. Certain subtypes, for example, are associated with more aggressive behavior and poorer outcomes, necessitating different therapeutic approaches compared to the classic form [15]. The latest WHO classification includes the classic, encapsulated, infiltrative follicular, diffuse sclerosing, solid/trabecular, Warthin-like, oncocytic, clear cell, spindle cell, PTC with fibromatosis/fasciitis-like/desmoid-type stroma, tall cell, hobnail, and columnar cell subtypes. Understanding the various subtypes of PTC is crucial for accurate diagnosis and tailored treatment strategies.
The classic PTC is characterized by a tumor with a papillary structure and cells exhibiting typical nuclear features. When a fibrous capsule surrounds this tumor, it is classified as the encapsulated subtype of PTC. The infiltrative follicular subtype of PTC is characterized by a tumor with an infiltrative pattern predominantly consisting of follicular structures. It has been highlighted that the encapsulated follicular variant of PTC (FVPTC) with invasion is excluded from this subtype and reclassified as a distinct entity due to different molecular alterations. The infiltrative follicular subtype of PTC is more akin to BRAF-like tumors, while the invasive encapsulated FVPTC is classified as RAS-like tumors. The oncocytic PTC is a rare subtype that shows a papillary structure lined by oncocytic cells with typical PTC nuclear features. Warthin-like PTC has a similar appearance to the Warthin tumor of the salivary gland but is composed of oncocytic cells exhibiting PTC nuclear features. This subtype usually occurs in the background of chronic lymphocytic thyroiditis. Clear cell PTCs should be differentiated from other secondary neoplasms including renal cell carcinoma. The diffuse sclerosing PTC shows squamous metaplasia, extensive lymphatic invasion, dense sclerosis, and numerous psammoma bodies in the background of Hashimoto thyroiditis. The tall cell PTC is characterized by a tumor comprising more than 30% of cells whose height is at least three times their width, exhibiting abundant eosinophilic cytoplasm and a prominent cell membrane. The clinical behavior of this subtype was more aggressive compared to classic PTCs. A diagnosis of the solid subtype of PTC requires the presence of a solid or trabecular growth pattern in more than 50% of the tumor mass. The hobnail PTC is rare and exhibits peculiar morphology, with tumor nuclei bulging from the apical surface and displaying pleomorphism. The columnar PTC is characterized by tumors with columnar cells, nuclear pseudostratification, and pale eosinophilic to clear cytoplasm. The chromatin is typically dark, and typical PTC nuclear features are only present focally.
The American Thyroid Association’s risk stratification system incorporates clinical and pathological data, and molecular alterations such as BRAF V600E and TERT promoter mutations. This system categorizes tumors into low, intermediate, and high-risk groups, providing tailored therapeutic recommendations [8]. PTCs such as the tall cell, hobnail, and columnar subtypes are considered aggressive and are categorized into the intermediate risk group.
The cytologic features for each morphologic subtype are detailed in Table 1.
Histologically, papillary structures lined by typical PTC nuclear features are crucial for diagnosing classic PTC. FNA samples from classic PTC show various papillary-like architectural patterns in different sizes, syncytial-like appearance (Fig. 1), and occasionally cellular swirls. True papillary architecture is defined by cells arranged in papillae containing a fibrovascular core and covered by atypical cells on the surface. Cellular swirls appear as concentric aggregates of 50–200 tumor cells with oval-shaped cells on the surface (Fig. 2). The long axis of the cells is oriented perpendicular to the radius of the swirls, often described as the “onion skin” pattern [22]. The papillary structures must be distinguished from hyperplastic or degenerative papillae, which show incomplete or absent fibrovascular cores with subfollicular formations [23].
Classic PTC cells are typically arranged in syncytial-like flat sheets or monolayers with nuclear enlargement, crowding, and molding. This feature is essential for distinguishing PTC from benign thyroid lesions [15]. Nuclear enlargement is defined as a size more than 1.5 times that of a normal nucleus, while nuclear elongation refers to a width exceeding 1.5 times that of a normal nucleus. Enlarged nuclei cause adjacent nuclei to deform, resulting in nuclear molding [22]. Nuclear crowding indicates that atypical cells are closely packed, while nuclear overlapping occurs when one nucleus covers another [22]. The nuclei typically exhibit pale, finely textured, and evenly distributed chromatin (powdery chromatin), in contrast to the nuclei of benign follicular cells that exhibit dark chromatin [15].
Typical nuclear features of PTC include intranuclear pseudoinclusions, pale nuclei, irregular nuclei with a ground-glass appearance, solitary or multiple micronucleoli, and nuclear grooves. Intranuclear pseudoinclusions and nuclear grooves reflect increased nuclear membrane plasticity in tumor cells, making their nuclei more easily deformed than those of normal thyroid cells (Fig. 3). Intranuclear pseudoinclusions are cytoplasmic invaginations into the nucleus, bounded by the nuclear membrane, and vary in shape and size [15]. The diagnostic intranuclear pseudoinclusions are surrounded by condensed chromatin and display the same color or texture as the cell’s cytoplasm, in contrast to nuclear bubbles or vacuoles that lack a clear boundary and vary in size and shape (Fig. 4). The vacuole size is at least one-quarter of the nucleus, with sharply defined membranes and no nuclear materials [15,24]. Nuclear grooves are invaginations of the nuclear membrane along the long axis of nuclei, more commonly seen in oval-shaped atypical cells than in round-shaped ones (Fig. 5) [15,25]. Nuclear grooves alone are not enough to definitively diagnose PTC, as they can also be present in other conditions such as lymphocytic thyroiditis and benign nodules. Therefore, a definitive diagnosis of PTC requires the presence of multiple characteristic features related to cell architecture and nuclear characteristics [15]. Psammoma bodies are concentric calcified bodies and are also commonly observed in classic PTC (Fig. 6). In FNA samples, psammoma bodies can be solitary, multiple, or attached to cells, and they are found less frequently than in histopathology samples [15,26]. While psammoma bodies are readily observed in conventional smears, they are often lost during the preparation process in liquid-based cytology. As a result, they may be more challenging to detect on glass slides in liquid-based preparations.
The FVPTC is the second most prevalent subtype of PTC, constituting a significant percentage of overall PTCs. FVPTC can manifest as either infiltrative or encapsulated forms. Histopathologically, FVPTC with an infiltrative growth pattern is characterized by a follicular growth pattern (micro-, normo-, or macro-follicular) with minimal papillary areas (< 1%) and nuclear features consistent with classic PTC (Fig. 7) [15,27]. Infiltrative FVPTC shares similarities with classic PTC, including a high frequency of lymph node metastases, risk of recurrence, and BRAF V600E mutation. Conversely, invasive encapsulated FVPTCs tend to exhibit better behavior with a significant tendency for RAS-like genetic profiles [17,28]. Due to its distinct molecular, phenotypic, and clinical characteristics compared to other PTC subtypes, the recent WHO classification distinguishes invasive encapsulated FVPTC from other PTC subtypes [16-18].
The cytological diagnosis of tumors with a follicular pattern is difficult for pathologists. These tumors can range from benign follicular adenomas to carcinomas, and they are typically classified as Bethesda category IV (follicular neoplasms). However, a conclusive diagnosis requires histopathological evidence of invasion of the capsule and blood vessels, which cannot be determined with cytology alone. Remember the following text:
Despite the recent WHO classification of tumors categorizing infiltrative FVPTC and invasive encapsulated FVPTC as distinct neoplastic entities, differentiating between the two in cytological smears remains quite challenging. This often leads to a diagnosis classified as Bethesda category IV (follicular neoplasm) or V (suspicious for malignancy) in these tumors [29]. However, several cytological characteristics must be considered when distinguishing between the two subtypes. Infiltrative FVPTC, akin to classic PTC, exhibits a significant prevalence of the BRAF V600E mutation. Consequently, infiltrative FVPTC is believed to have more distinct and well-defined nuclear characteristics, resulting in a higher total nuclear score than the subtle nuclear features found in invasive encapsulated FVPTC with RAS-like genetic profile [30]. The distribution of nuclear features in infiltrative FVPTC is typically more diffuse than the focal nuclear features displayed in encapsulated types. Higher cellularity also serves as a distinguishing feature that differentiates infiltrative FVPTC from the relatively indolent invasive encapsulated FVPTC [30]. Invasive encapsulated FVPTC typically reveals microfollicular structures with smaller, round to oval nuclei, nuclear overlapping, and darker chromatin [29].
FNA aspirates of the FVPTC with infiltrative growth patterns may predominantly exhibit microfollicular cell clusters consisting of small to medium-sized follicles, occasionally accompanied by dense globus colloids in the background. These patterns can mimic other follicular neoplasms. Key features include increased tumor cellularity, follicles or syncytial clusters, and the prominent variable nuclear features characteristic of classic PTC [15,27,31,32]. Notably, FVPTC lacks papillary structures and psammoma bodies [33]. The nuclear features frequently associated with FVPTC include prominent nuclear grooves along the long axis of the nuclei [31], nuclear enlargement, nuclear elongation, and finely dispersed chromatin (powdery chromatin) [15,32]. Although nuclear crowding and intranuclear pseudoinclusions may occasionally be observed, they are typically limited compared to classic PTC [33].
Despite a meta-analysis study [34] has shown significant differences in the cytological features of FVPTC and noninvasive follicular thyroid neoplasm with papillary-like nuclear feature (NIFTP), distinguishing between the two entities based solely on cytology remains challenging in clinical practice. Statistically, NIFTP tends to show a higher prevalence of microfollicles, while FVPTC is more frequently associated with marginal nucleoli, multinucleated giant cells, nuclear elongation, nuclear enlargement, nuclear grooves, and intranuclear inclusions [35]. Furthermore, NIFTP is characterized by a lack of nuclear crowding, smoother nuclear grooves, and smaller nuclei, less elongated and rounder than those seen in classic PTC. It is also crucial to emphasize that cytological examination alone cannot differentiate FVPTC from NIFTP, as the distinction is based on the presence of invasion, which cannot be assessed in FNA aspirates. As a result, the diagnostic sensitivity of FNA for identifying the FVPTC is lower than that for the classic PTCs [15].
In comparison to the FVPTC, follicular neoplasm (Bethesda IV) often displays an image of mild to moderate cellularity with microfollicle structure that includes ≤15 cells arranged in a flat circle that is at least two-thirds complete, with a lumen. Other differentiating features of follicular neoplasm are monotonous cell population and normal-sized or enlarged, relatively uniform follicular cells. The background may present a scant or absent colloid. Benign follicular lesions can be distinguished by their monolayered cellular arrangement that can be present as a honeycomb appearance, with well-defined cytoplasmic borders, regularly spaced cytoplasm, and uniform nuclear polarity. Thyroid spherules can resemble microfollicles, but they are only found in benign follicular nodular disease (Fig. 8) [36].
A presumed FVPTC, particularly those that display a solely follicular architectural pattern with scarce PTC nuclear features of intranuclear pseudoinclusion and psammoma bodies, should be regarded as suspicious for malignancy instead of being classified as outright malignant (category VI). This approach to interpretation is considered more appropriate in such cases to avoid overdiagnosis and helps maintain a high positive predictive value for malignancy in suspicious for malignancy (category V).
FNA materials from tall cell PTCs typically exhibit high cellularity, with tumor cells arranged in a palisading or parallel pattern on one side of the cluster, mimicking the tram-tracked pattern observed histologically. Tall cell PTC is characterized by tall columnar tumor cells with oncocytic features, and centrally located nuclei, which may elongate and become cylindrical with eccentric nuclei (resembling tail-like or tadpole cells). The nuclear chromatin is less powdery and more granular, with prominent, centrally located nucleoli. The cell cytoplasm is abundant, granular eosinophilic, or cyanophilic with prominent cell membranes (Fig. 9). Definitive diagnosis requires the identification of PTC nuclear characteristics. Intranuclear pseudoinclusions are more commonly observed and can appear multiple within a single tumor cell nucleus, giving a “soap bubble” appearance. When tumor cells form cohesive clusters, tall cells are identified at the cluster margin; however, if the clusters are loose or non-cohesive, the morphology of the tall cells is more apparent. The tumor background usually consisted of minimal colloid and inflammatory cells. Psammoma bodies are infrequently observed in this subtype [12,15,37].
The columnar cell PTC is an uncommon but aggressive subtype, representing less than 0.3% of all PTC cases [38-40]. The tumor is typically large and invasive, with a higher prevalence in females and older adults [39]. The subtype commonly exhibits aggressive clinical features, such as local invasion, lymph node metastasis, and distant metastasis. However, a smaller subset of well-circumscribed tumors that arise in younger individuals tend to exhibit a relatively more indolent behavior [40]. Histologically, columnar cell PTC is defined by papillae or glandular-like structures lined by columnar cells that display prominent nuclear pseudostratification, occasionally accompanied by supranuclear or subnuclear vacuoles [38]. Due to its rarity, there is a paucity of studies focused on the cytomorphological features of this subtype, which presents challenges in columnar cell PTC subtyping diagnosis.
The cytomorphological features of columnar cell PTC, akin to other aggressive PTC subtypes, are attributed to epithelial-mesenchymal transition [41]. This phenomenon causes a loss of cellular polarity, which changes the nuclear position within the cytoplasm, leading to nuclear pseudostratification. In FNA samples, columnar cell PTC often presents as hypercellular smears with an architectural arrangement of pseudostratified layers or glandular structures lined by columnar cells [15,41]. The hallmark of nuclear features may include an enlarged, hyperchromatic, stratified nucleus with abundant basophilic cytoplasm. There may be occasional PTC nuclear features such as nuclear crowding, intranuclear pseudoinclusions, and nuclear grooves, although they are often very scarce/absent. The background is typically clean, with occasional findings of mucinous material [38]. The overall morphology of columnar cell PTC may closely resemble that of adenocarcinoma of the endometrium or gastrointestinal tract.
The cytomorphology of columnar cell PTC may mimic that of the tall cell PTC due to similar elongated nuclear features. Both subtypes can exhibit a tram-track pattern; however, columnar cell PTC can be distinguished by its clear cytoplasm, as opposed to the oncocytic-like cytoplasm typically observed in tall cell PTC and the presence of nuclear pseudostratification. In contrast, the tall cell PTC is characterized by bright eosinophilic cytoplasm and more pronounced nuclear features, including nuclear grooves and abundant intranuclear pseudoinclusions [12]. The pseudostratified arrangement of columnar cell PTC may also resemble the upper respiratory epithelium, sometimes unintentionally collected during thyroid aspirations. Key distinguishing factors of columnar cell PTC are the absence of cilia in any of the cells and the occasional, subtle manifestation of PTC nuclear features [41].
Additional molecular testing may also be helpful. Like tall cell and hobnail PTCs, columnar cell PTC has a high BRAF V600E proportion [42]. However, a recent publication by Higgins et al. [43] discovered that columnar cell PTC is a heterogeneous entity consisting of similar proportions of RAS-like and BRAF-like genetic profiles.
The hobnail PTC is rare, but it tends to exhibit aggressive behavior with a tendency for distant metastasis, poor response to radioiodine uptake, and high mortality rates. This tumor may appear as a true hobnail subtype or a hobnail-like PTC. It is important to differentiate between the two because they have distinct prognoses [16].
In contrast to the true papillae found in classic PTC, hobnail PTC is invasive and displays a complex micropapillary growth pattern without a true vascular core in the papillae. The lack of cohesiveness in this subtype suggests a possible role in metastasis associated with epithelial-mesenchymal transition. The tumor background often shows dense fibrosis and inflammation. The hobnail cells still retain the nuclear features of PTC [44].
Cytologically, the aspirate is typically highly cellular, with tumor cells dispersed as single cells or arranged in micropapillary clusters and occasionally papillary structures. A follicular pattern is rarely observed in this tumor. The colloid is usually scant, often accompanied by a bloody or cystic background. The tumor cells are medium-sized, exhibiting characteristic hobnail morphology with nuclei protruding from the apical surface, crowded cellular arrangement, high nuclear-to-cytoplasmic ratio, prominent nucleoli, dense chromatin, and a rather discohesive appearance. The tumor cells retain the nuclear features of PTC, such as nuclear grooves and soap bubble-like intranuclear pseudoinclusions, although they may show more pronounced nuclear atypia and pleomorphism [44]. The cytoplasm is frequently scant, ranging from clear to eosinophilic. Tumor cells that are arranged as scattered single cells may appear comet-like or tear-drop-like appearance due to tapered cytoplasm (Fig. 10) [45]. The classic PTC and tall cell PTC serve as differential diagnoses for this entity due to eccentric nuclei, abundant cytoplasm, and prominent cell membranes. However, the absence of characteristic hobnail or comet-like features excludes these subtypes [44].
FNA suggesting hobnail PTC may influence a shift towards a more aggressive management approach. According to the WHO classification, at least 30% of the tumor must exhibit a hobnail component to be classified as the hobnail subtype. However, even a hobnail component as low as 10% is associated with a poorer prognosis. Thus, any presence of a hobnail component should be meticulously noted in the pathology report [46].
The diffuse sclerosing PTC typically occurs at a younger age and carries a higher risk of extrathyroidal extension, lymph node, and distant metastases than classic PTC [47-50]. The combination of sonogram and cytology is useful for diagnosing diffuse sclerosing PTC preoperatively. The sonogram typically shows a “snowstorm” pattern and is often present as hypoechoic solid nodules. However, the sonogram of Hashimoto’s thyroiditis may show a heterogeneous background that mimics diffuse sclerosing PTC. This finding may mislead clinicians and delay the diagnosis [51].
The tumor may appear as a nodule or non-nodular macroscopically. The histopathologic features are unique, characterized by classic PTC diffusely involving the thyroid gland, with squamous metaplasia and psammoma bodies within a background of dense sclerotic stroma associated with chronic lymphocytic thyroiditis and dilated lymphatic spaces. Several patterns can indicate diffuse sclerosing PTC cytologically. Besides monolayered papillary structures, solid ball-like clusters are common and characterized by tumor cells arranged in spherical or three-dimensional clusters. Mirror ball or hollow ball clusters containing faint septate cytoplasmic vacuoles and large unilocular vacuoles, or with squamous features, are also seen [52].
The tumor cells were polygonal or low columnar and mostly did not show a ground-glass appearance. The chromatin pattern of this subtype is mostly coarser than that of classic PTC. Intranuclear pseudoinclusions and nuclear grooves may be seen. Some cells may appear hobnail-like with scant to moderate dense cytoplasm with squamoid or metaplastic changes. The tumor background is characterized by many lymphocytes and foamy histiocytes with psammoma bodies (Fig. 11). Ropy colloids could be found. These cytological characteristics help differentiate diffuse sclerosing PTC from benign conditions such as Riedel’s and Hashimoto’s thyroiditis [52,53].
The prevalence of Warthin-like PTC ranges from 0.2% to 1.9% of all PTC cases [15]. This subtype predominantly affects women and tends to occur in younger ages, typically between 30 and 50 years of age, compared to classic PTC. Prognostically, Warthin-like PTC generally follows a favorable course, with infrequent extrathyroidal extension, low recurrence rates, and rare lymph node metastasis. Furthermore, the tumor is associated with the BRAF V600E mutation in 65%–75% of cases. The 20-year survival rate for Warthin-like PTC exceeds 90%, although some cases demonstrate more aggressive behavior [54-56].
Cytomorphologically, Warthin-like PTC is distinguished by the presence of tumor cells organized in monolayers with papillary structures, along with a subset of tumor cells that are dispersed [15,57]. The follicular cells exhibit oncocytic features, including large, polygonal shapes, inconspicuous nucleoli, and abundant granular eosinophilic cytoplasm. The nuclear features of classic PTC, such as nuclear enlargement, nuclear crowding, powdery chromatin, and nuclear grooves, are also present [15,55,57]. The stroma in Warthin-like-PTC is characterized by fibrovascular stalks infiltrated with lymphocytes and plasma cells, resulting in a lymphoplasmacytic background. Intranuclear inclusions are more readily identified in liquid-based cytology due to the relatively high cellularity, whereas lymphoplasmacytic background and papillary structures are more prominent in conventional smears [54,57].
The differential diagnoses of Warthin-like PTC include oncocytic PTC, classic PTC with concurrent Hashimoto thyroiditis, oncocytic carcinoma of the thyroid, and Hashimoto thyroiditis. In cases where the papillary architecture and nuclear features of classic PTC are not well-defined, Warthin-like PTC may be misdiagnosed as a follicular adenoma or follicular carcinoma with oncocytic features [15,54,57]. According to Kim et al. [54] in comparison to classic PTC without thyroiditis, Warthin-like PTC identified on FNA cytology exhibits a greater abundance of lymphocytes and histiocytes within the tumor clusters as well as in the background of the tumor [56]. Unlike the oncocytic PTC, Warthin-like PTC exhibits lymphoplasmacytic infiltration, though differentiating these entities caries minimal clinical implications for management or prognosis [15,54]. Differentiating Warthin-like PTC from Hashimoto thyroiditis on FNA cytology requires attention to oncocytic cell characteristics, as Hashimoto thyroiditis typically presents with round nuclei and prominent single nucleoli, whereas Warthin-like PTC displays irregular nuclear contours and inconspicuous nucleoli [15]. However, the limitations of FNA cytology necessitate histopathological examination as the gold standard for the definitive diagnosis Warthin-like PTC [55,56].
The solid/trabecular PTC represents 1%–3% of adult cases and occurs more frequently in pediatric patients [58]. Classically, this subtype has been closely associated with radiation exposure, with over 30% of children affected by the Chernobyl disaster developing this cancer [58]. However, recent studies have shown that solid/trabecular PTC can also occur sporadically and is associated with a heterogeneous genetic profile, including RET gene fusions, BRAF V600E, and TERT promoter mutations [58].
Histologically, solid/trabecular PTC is defined by solid, trabecular, or nested cellular growth pattern, which account for more than 50% of the tumor and exhibit typical PTC nuclear features [16,17]. The presence of this growth pattern is significantly associated with higher rates of vascular invasion [59], tumor recurrence, and mortality compared to classic PTC [58]. However, due to the lack of distinctive cytological markers, diagnosing this subtype based solely on cytology is challenging. Most cases are typically classified as suspicious for malignancy (Bethesda V) or simply as PTC [60].
In FNA samples, the solid/trabecular PTC often presents moderately to highly cellular smears in a clean background with minimal colloid [15,41]. The neoplastic cells typically form solid or trabecular growth patterns, lacking the papillary or follicular structures commonly seen in other subtypes. The nuclei exhibit typical PTC nuclear features with prominent nucleoli, while the cytoplasm of these cells is usually moderate to abundant and eosinophilic [41]. Additionally, the presence of loosely or non-cohesive single cells is indicative of the infiltrative and aggressive nature of this tumor [15]. Compared with the classic PTC, the nuclear atypia in solid/trabecular PTC is particularly more pronounced with nuclear overlapping, pleomorphism, and a higher nuclear-to-cytoplasm ratio [41]. In contrast, the presence of psammoma bodies is very rare in solid/trabecular PTC [41].
The occasional presence of microfollicular structures may lead to diagnostic challenges in differentiating the solid/trabecular PTC from follicular-patterned neoplasm [59]. Follicular-patterned neoplasm typically exhibits a monolayered cellular architecture. In contrast, the solid/trabecular PTC is distinguished by its characteristic three-dimensional cellular architectures, forming syncytial structures in cytological samples [41]. Despite the growth pattern observed in solid/trabecular PTC, it should not be conflated with DHGTC and PDTC, as solid/trabecular PTC lacks the features of tumor necrosis and a high mitotic rate that are characteristic of DHGTC and PDTC. PDTC typically shows no nuclear groove or intranuclear pseudoinclusion, which is typical of PTC.
The oncocytic PTC comprises approximately 1%–11% of all PTC cases. It predominantly affects females, with a reported female-to-male ratio of 4:1. Classically, oncocytic PTC has been associated with poor prognostic outcomes [61]. However, with the implementation of more stringent histological diagnostic criteria and the advent of radioiodine therapy, recent studies have increasingly reported a more favorable prognosis [62], with 5-year and 10-year survival rates of 93% and 81%, respectively. Local recurrence and distant metastasis are infrequent, occurring in fewer than 10% of cases [62-64].
Cytologically, the oncocytic PTC is characterized by a tumor architecture comprising papillary structures, sheets, microfollicles, or individual dispersed cells, with more than 75% of the tumor cells exhibiting oncocytic features [15,63]. Oncocytic cells are polygonal with abundant granular cytoplasm due to mitochondrial accumulation, which reflects an adaptive response of thyroid follicular cells to primary mitochondrial DNA alterations. The presence of nuclear features characteristic of classic PTC is crucial for the definitive diagnosis of oncocytic PTC [15,63]. Tumor necrosis and increased mitotic activity are typically absent [63]. The lymphocytic background is either minimal or absent in the oncocytic PTC [15].
The diagnostic accuracy of FNA for oncocytic PTC is limited, with a reported precision rate of approximately 60%. This limitation arises from numerous differential diagnoses associated with oncocytic lesions [64]. The oncocytic PTC can resemble other oncocytic proliferations, such as those seen in Hashimoto thyroiditis, oncocytic medullary thyroid carcinoma, and oncocytic neoplasms originating from other organs, including metastatic renal cell carcinoma. Distinguishing the oncocytic PTC from other oncocytic lesions requires careful evaluation of oncocytic cell morphology. In non-oncocytic PTC, oncocytic cells are typically round with prominent nucleoli, minimal nuclear grooves, paler nuclear staining, and infrequent intranuclear inclusions [15]. In Hashimoto thyroiditis, oncocytic cells exhibit greater cohesiveness, anisokaryosis, and lack nucleoli [64]. In contrast, the oncocytic PTC is characterized by classic PTC nuclear features, abundant oncocytic cells, and papillary structures with fibrovascular stalks [15,64]. However, when nuclear features of the oncocytic PTC are not well-defined, the diagnosis may be classified as a follicular neoplasm, an oncocytic follicular neoplasm, or suspicious for an oncocytic PTC. If a significant lymphocytic infiltration is observed in the tumor background, consideration should be given to the diagnosis of Warthin-like PTC [15].
The increasing incidence of thyroid cancer, particularly PTC, has been significantly driven by advancements in diagnostic modalities such as ultrasound and FNA [65]. PTC remains the most prevalent form of thyroid malignancy, characterized by a generally favorable prognosis, with a 10-year survival rate exceeding 90%, especially when detected early. However, recurrence is observed in approximately 7%–23% of PTC cases. Recurrence risk factors include age, sex, tumor size, extrathyroidal extension, distant metastasis, lymphovascular invasion, and lymph node metastasis, particularly involving cervical and paratracheal lymph nodes. Among these, lymph node metastasis represents a critical risk factor for recurrence [66]. Cervical lymph node metastasis is detected in 40%–60% of PTC cases, particularly among younger patients, which aligns with findings from Liu et al. [67]. This study indicates an association between lymph node metastasis, younger age, poorer prognosis, and decreased life expectancy [68]. PTC patients younger than 45 years, with metastasis to more than five lymph nodes, exhibit a higher mortality rate [69].
Lymph node metastasis is common in epithelial malignancies, with tumor cells infiltrating lymphatic vessels and spreading to regional lymph nodes to form metastatic foci [69]. In addition to lymphatic invasion, several histologic features are associated with lymph node metastasis, including the presence of psammoma bodies, micropapillary architecture, and distinct subtypes such as hobnail and tall cell PTCs [70]. The clinical guidelines recommend preoperative neck ultrasounds with FNA of suspicious cervical lymph nodes, particularly in patients with malignant or suspected malignant thyroid FNA results. The combination of FNA and ultrasounds approach offers high specificity and sensitivity in detecting lymph node metastasis in thyroid carcinoma [70]. This approach aims to reduce clinical management errors [70].
The presence of PTC tumor cells in lymph nodes is definitive evidence of metastasis, with characteristics metastatic features including monolayer or papillary structures, powdery chromatin nuclei, intranuclear pseudoinclusions, and nuclear grooves. Additional findings may include multinucleated giant cells, foamy histiocytes, colloid, and psammoma bodies [71]. However, these features may not always be observed in FNA specimens from lymph nodes with suspected metastasis [72].
Cystic degeneration is frequently observed in PTC (Fig. 12), both in primary tumors and in metastatic lymph nodes [72]. FNA of lymph nodes revealing cystic degeneration without identifiable tumor cells is more commonly associated with PTC metastasis than with primary tumors [71,73]. Cystic degeneration in lymph nodes can also occur in metastases from salivary gland tumors, highlighting the importance of confirming the thyroid origin of such changes. To reduce the risk of false-negative diagnoses, immunohistochemical and biochemical markers such as cytokeratin 19 and thyroglobulin can be utilized to confirm that cystic degeneration is of thyroid origin. However, this approach is limited to fresh cyst fluid aspirates or cell block preparations [73].
In addition to cystic degeneration, pigmented foamy histiocytes are frequently observed in PTC metastasis to lymph nodes. A study by Ng et al. [73] demonstrated the presence of pigmented foamy histiocytes, even in the absence of tumor cells in lymph node FNA, correlated with positive histopathological findings of PTC metastasis. Histopathological examination of PTC typically reveals not only the characteristic nuclear features of classic PTC but also calcification, multinucleated giant cells, and colloidal material. These features, which are commonly linked to a positive diagnosis of PTC metastasis, can also be observed in lymph node FNA. The presence of colloid alone in lymph node FNA cytology can serve as a marker of PTC metastasis.
In addition to lymph node FNA, Choi et al. [70] suggested that certain findings in preoperative thyroid FNA could predict the likelihood of PTC metastasis to lymph nodes. Specifically, the presence of atypical histiocytoid cells and multinucleated giant cells may indicate an increased risk of metastasis. Atypical histiocytoid cells, which resemble histiocytes or epithelioid cells, with hyperchromatic to vesicular nuclei and abundant cytoplasm, are often found in PTC with discohesive tumor cell structures or micropapillary arrangements, both of which are correlated with lymph node metastasis. Multinucleated giant cells, which vary in size and number, are associated with PTCs that exhibit extrathyroidal extension, larger tumor size, and lymph node metastasis. Both atypical histiocytoid cells and multinucleated giant cells (Fig. 13) are more frequently found in PTC cases with moderate to high cellularity, which further correlates with an increased likelihood of lymph node metastasis. Therefore, the presence of these cells in thyroid FNA may serve as a predictive marker for PTC metastasis to lymph nodes before surgery [70].
Lymph node FNA is known to have a high false-negative rate, estimated to be around 30%, primarily due to sampling errors. Normal lymph node FNA specimens typically contain lymphoid fragments, germinal centers, and a background of abundant inflammatory cells, which serve as indicators of sample adequacy. The identification of these components may help reduce false-negative rates. According to Ng et al. [73], FNA specimens that contained normal lymph node components had a false-negative rate of 18.8%, compared to 72.2% in FNA specimens lacking these components.
DHGTC is a novel entity that has emerged in the latest WHO classification edition due to its significant clinical behavior. This entity is classified as a high-grade follicular cell-derived non-anaplastic thyroid carcinoma, along with PDTC, exhibiting an intermediate prognosis between well-differentiated thyroid carcinomas and ATC [16-18]. Patients with DHGTC often have a poor prognosis due to the high likelihood of distant metastasis and resistance to conventional therapies [74].
DHGTC refers to well-differentiated thyroid carcinomas (PTC, FTC, or oncocytic carcinoma) with high-grade features, defined by the presence of ≥ 5 mitoses per 2 mm 2 or tumor necrosis. The aggressive nature of DHGTC necessitates early and accurate preoperative diagnosis to guide appropriate management. A definitive diagnosis of DHGTC cannot be established from cytology alone; however, suspicion of this entity can alter patient management strategies. The cytological features of DHGTC are challenging because they still contain components of well-differentiated thyroid carcinomas and lead to inconsistent Bethesda classifications depending on the underlying tumor type. Tondi Resta et al. [75] reviewed 32 cases of DHGTC cytologically, showing that 43.8% of the cases were diagnosed as Bethesda category VI (malignant) and 31.3% as follicular neoplasm. Most cases diagnosed as Bethesda category VI were DHGTC originating from the PTC in histopathological specimens. Most cases of DHGTC originating from tumors with oncocytic differentiation, such as the oncocytic PTC and oncocytic carcinoma of the thyroid, were diagnosed under the Bethesda category IV.
Cytologically, the FNA shows highly cellular specimens with crowded and overlapping features. Some tumor cells may be arranged in small groups, clusters, or papillary structures with a fibrovascular core. The tumor cells exhibit a syncytial arrangement with abundant cytoplasm and indistinct cell borders. The nuclei are round to irregular, significant atypia with coarse chromatin and sometimes prominent nucleoli. Mitosis can be found, while necrosis or karyorrhexis debris may be present in the background. The presence of well-differentiated thyroid carcinomas with such findings is crucial, raising suspicion of this entity [41]. The main differential diagnosis for this entity is PDTC. Several cytological features are associated with PDTC, including highly cellular smears with solid/insular architecture of monomorphic follicular cells, high nuclear/cytoplasmic ratio, nuclear atypia, irregular membrane so-called convoluted nuclei, scanty cytoplasm, and the presence of mitosis, apoptosis, or necrosis (Fig. 14). However, these findings are not specific [15]. The existence of PDTC cases that still contain well-differentiated thyroid carcinoma elements complicates the differentiation from DHGTC.
PTC encompasses a wide range of cytologic and histologic features that are crucial for accurate diagnosis, prognosis, and treatment. FNA cytology is the primary method for evaluating thyroid nodules, including those related to PTC. However, diagnosing different PTC subtypes can be challenging due to overlapping features and the presence of rare and aggressive subtypes. This requires a careful and nuanced approach to diagnosis.
This review focused on the distinct cytologic characteristics of various PTC subtypes, such as classic, follicular, tall cell, columnar cell, hobnail, diffuse sclerosing, Warthin-like, solid/trabecular, and oncocytic subtypes. It underscores the importance of recognizing their unique nuclear features, architectural patterns, and background elements in order to differentiate them from benign thyroid conditions and other thyroid malignancies.
Accurate cytologic diagnosis is particularly crucial for aggressive subtypes like tall cell, hobnail, and columnar cell PTCs, as they have different prognostic and therapeutic implications. The review also explored cytologic indicators of tumor aggressiveness, such as lymph node metastasis and features associated with high-grade thyroid carcinoma.
It emphasizes the need for careful cytologic evaluation and the potential role of ancillary techniques, such as molecular testing, to improve diagnostic accuracy. Looking ahead, standardized cytologic criteria and advanced diagnostic techniques, including imaging and molecular profiling, are needed to classify PTC subtypes better and predict their clinical behavior. These advancements will likely lead to more personalized and effective management strategies for thyroid cancer patients.
Continuous education and experience are essential for cytopathologists to overcome diagnostic challenges and minimize the risk of misclassification, ultimately improving patient outcomes.
Notes
Availability of Data and Material
All data analyzed during this study are included in this published article.
Author Contributions
Conceptualization: CKJ. Data curation: CKJ, ASH. Formal analysis: CKJ, ASH. Funding acquisition: CKJ. Investigation: CKJ. Methodology: ASH, CKJ. Project administration: CKJ. Resources: CKJ. Supervision: CKJ. Validation: ASH, CKJ. Visualization: ASH, CKJ. Writing—original draft: ASH, CKJ. Writing—review & editing: ASH, CKJ. Approval of final manuscript: all authors.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024; 74:229–63.

2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72:7–33.

3. Dal Maso L, Tavilla A, Pacini F, et al. Survival of 86,690 patients with thyroid cancer: a population-based study in 29 European countries from EUROCARE-5. Eur J Cancer. 2017; 77:140–52.
4. Samankan S, Militello L, Seo G, et al. Tall cell variant papillary thyroid carcinoma impacts disease-free survival at the 10 % cut-point on multivariate analysis. Pathol Res Pract. 2022; 236:154012.

5. Lee JS, Lee JS, Yun HJ, et al. Aggressive subtypes of papillary thyroid carcinoma smaller than 1 cm. J Clin Endocrinol Metab. 2023; 108:1370–5.

6. Vuong HG, Long NP, Anh NH, et al. Papillary thyroid carcinoma with tall cell features is as aggressive as tall cell variant: a meta-analysis. Endocr Connect. 2018; 7:R286–93.

7. Parvathareddy SK, Siraj AK, Annaiyappanaidu P, et al. Risk factors for cervical lymph node metastasis in Middle Eastern papillary thyroid microcarcinoma. J Clin Med. 2022; 11:4613.

8. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.
9. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, Vander-Laan PA. The 2023 Bethesda System for Reporting Thyroid Cytopathology. J Am Soc Cytopathol. 2023; 12:319–25.

10. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009; 19:1159–65.

11. Lee EK, Park YJ, Jung CK, Na DG. A narrative review of the 2023 Korean Thyroid Association management guideline for patients with thyroid nodules. Endocrinol Metab (Seoul). 2024; 39:61–72.

12. Canberk S, Montezuma D, Ince U, et al. Variants of papillary thyroid carcinoma: an algorithmic cytomorphology-based approach to cytology specimens. Acta Cytol. 2020; 64:288–98.

13. Rossi ED, Martini M, Capodimonti S, et al. Diagnostic and prognostic value of immunocytochemistry and BRAF mutation analysis on liquid-based biopsies of thyroid neoplasms suspicious for carcinoma. Eur J Endocrinol. 2013; 168:853–9.

14. Nikiforov YE, Carty SE, Chiosea SI, et al. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid. 2015; 25:1217–23.

15. Pusztaszeri M, Stelow E, Westra W, Zakowski M, Mastorakis E. Papillary thyroid carcinoma, subtypes, and related tumors. In: Ali SZ, VanderLaan PA, eds. The Bethesda System for Reporting Thyroid Cytopathology. Cham: Springer Nature;2023. p. 135–76. 3rd.
16. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul). 2022; 37:703–18.

17. Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022; 33:27–63.

18. Bychkov A, Jung CK. What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system. J Pathol Transl Med. 2024; 58:98–101.

19. Goemann IM, Romitti M, Meyer EL, Wajner SM, Maia AL. Role of thyroid hormones in the neoplastic process: an overview. Endocr Relat Cancer. 2017; 24:R367–85.

20. Fagin JA, Nikiforov YE. Progress in thyroid cancer genomics: a 40-year journey. Thyroid. 2023; 33:1271–86.

21. Nikiforov YE, Biddinger PW, Thompson LD. Diagnostic pathology and molecular genetics of the thyroid: a comprehensive guide for practicing thyroid pathology. Philadelphia: Wolters Kluwer;2020. p. 249–50. 3rd.
22. Xiong XJ, Xiao MM, Zhang YX, et al. The accurate interpretation and clinical significance of morphological features of fine needle aspiration cells in papillary thyroid carcinoma. Anal Cell Pathol (Amst). 2023; 2023:9397755.

23. LiVolsi VA, Baloch Z. Noninvasive follicular tumor with papillary-like nuclear features: a practice changer in thyroid pathology. Arch Pathol Lab Med. 2021; 145:659–63.

24. Schwertheim S, Theurer S, Jastrow H, et al. New insights into intranuclear inclusions in thyroid carcinoma: association with autophagy and with BRAFV600E mutation. PLoS One. 2019; 14:e0226199.

25. Ashwini BR, Nirmala C, Natarajan M, Biligi DS. A study to evaluate association of nuclear grooving in benign thyroid lesions with RET/PTC1 and RET/PTC3 gene translocation. Thyroid Res. 2023; 16:21.

26. Henke LE, Pfeifer JD, Baranski TJ, DeWees T, Grigsby PW. Long-term outcomes of follicular variant vs classic papillary thyroid carcinoma. Endocr Connect. 2018; 7:1226–35.

27. Trabzonlu L, Paksoy N. Cytomorphological analysis of thyroid nodules diagnosed as follicular variant of papillary thyroid carcinoma: a fine needle aspiration study of diagnostic clues in 42 cases and the impact of using Bethesda system in reporting: an institutional experience. Endocr Pathol. 2018; 29:351–6.

28. Kim C, Agarwal S, Bychkov A, et al. Differentiating BRAF V600E-and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study. Virchows Arch. 2024; 484:645–56.

29. Yang GC, Fried KO, Scognamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: a review of 179 cases. Diagn Cytopathol. 2017; 45:533–41.
30. Basolo F, Macerola E, Poma AM, Torregrossa L. The 5(th) edition of WHO classification of tumors of endocrine organs: changes in the diagnosis of follicular-derived thyroid carcinoma. Endocrine. 2023; 80:470–6.

31. Ibrahim AA, Wu HH. Fine-needle aspiration cytology of noninvasive follicular variant of papillary thyroid carcinoma is cytomorphologically distinct from the invasive counterpart. Am J Clin Pathol. 2016; 146:373–7.

32. Yoon JH, Kwon HJ, Kim EK, Moon HJ, Kwak JY. The follicular variant of papillary thyroid carcinoma: characteristics of preoperative ultrasonography and cytology. Ultrasonography. 2016; 35:47–54.

33. Maleki S, Zandvakili A, Gera S, Khutti SD, Gersten A, Khader SN. Differentiating noninvasive follicular thyroid neoplasm with papillary-like nuclear features from classic papillary thyroid carcinoma: analysis of cytomorphologic descriptions using a novel machine-learning approach. J Pathol Inform. 2019; 10:29.

34. Haaga E, Kalfert D, Ludvikova M, Kholova I. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features is not a cytological diagnosis, but it influences cytological diagnosis outcomes: a systematic review and meta-analysis. Acta Cytol. 2022; 66:85–105.

35. Na HY, Park SY. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: its updated diagnostic criteria, preoperative cytologic diagnoses and impact on the risk of malignancy. J Pathol Transl Med. 2022; 56:319–25.

36. Sohn H, Kakudo K, Jung CK. Diagnostic implication of thyroid spherules for cytological diagnosis of thyroid nodules. Cytopathology. 2024; 35:383–9.

37. Lee SH, Jung CK, Bae JS, Jung SL, Choi YJ, Kang CS. Liquid-based cytology improves preoperative diagnostic accuracy of the tall cell variant of papillary thyroid carcinoma. Diagn Cytopathol. 2014; 42:11–7.

38. Bongiovanni M, Mermod M, Canberk S, et al. Columnar cell variant of papillary thyroid carcinoma: cytomorphological characteristics of 11 cases with histological correlation and literature review. Cancer Cytopathol. 2017; 125:389–97.

39. Wenig BM, Thompson LD, Adair CF, Shmookler B, Heffess CS. Thyroid papillary carcinoma of columnar cell type: a clinicopathologic study of 16 cases. Cancer. 1998; 82:740–53.
40. Silver CE, Owen RP, Rodrigo JP, Rinaldo A, Devaney KO, Ferlito A. Aggressive variants of papillary thyroid carcinoma. Head Neck. 2011; 33:1052–9.

41. Kakudo K, Liu Z, Jung CK, Hirokawa M, Bychkov A, Lai CR. Thyroid FNA cytology: differential diagnoses and pitfalls. Singapore: Springer Singapore;2023. p. 149–427. 3rd.
42. Janovitz T, Williamson DF, Wong KS, Dong F, Barletta JA. Genomic profile of columnar cell variant of papillary thyroid carcinoma. Histopathology. 2021; 79:491–8.

43. Higgins KE, Sadow PM, Johnson DN, Wang P, Wanjari P, Cipriani NA. Columnar cell thyroid carcinoma: a heterogeneous entity demonstrating overlap between papillary thyroid carcinoma and follicular neoplasms. Head Neck Pathol. 2024; 18:39.

44. Ambrosi F, Righi A, Ricci C, Erickson LA, Lloyd RV, Asioli S. Hobnail variant of papillary thyroid carcinoma: a literature review. Endocr Pathol. 2017; 28:293–301.

45. Bellevicine C, Cozzolino I, Malapelle U, Zeppa P, Troncone G. Cytological and molecular features of papillary thyroid carcinoma with prominent hobnail features: a case report. Acta Cytol. 2012; 56:560–4.

46. Wong KS, Chen TY, Higgins SE, et al. A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology. 2020; 76:707–13.

47. Malandrino P, Russo M, Regalbuto C, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid. 2016; 26:1285–92.

48. Pillai S, Gopalan V, Smith RA, Lam AK. Diffuse sclerosing variant of papillary thyroid carcinoma: an update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol. 2015; 94:64–73.

49. Cavaco D, Martins AF, Cabrera R, Vilar H, Leite V. Diffuse sclerosing variant of papillary thyroid carcinoma: outcomes of 33 cases. Eur Thyroid J. 2022; 11:e210020.

50. Kim SY, Shin SJ, Lee DG, et al. Clinicopathological and genetic characteristics of patients of different ages with diffuse sclerosing variant papillary thyroid carcinoma. Cancers. 2023; 15:3101.

51. Li W, Wang Y, Gao L, et al. Sonographic characteristics of diffuse sclerosing variant of papillary thyroid carcinoma with histopathological correlation: a preliminary study. Orphanet J Rare Dis. 2024; 19:136.

52. Takagi N, Hirokawa M, Nobuoka Y, Higuchi M, Kuma S, Miyauchi A. Diffuse sclerosing variant of papillary thyroid carcinoma: a study of fine needle aspiration cytology in 20 patients. Cytopathology. 2014; 25:199–204.

53. Houas J, Ghammam M, Chouchane L, et al. An unusual presentation of diffuse sclerosing variant of papillary thyroid carcinoma. Egypt J Otolaryngol. 2022; 38:78.

54. Kim J, Lim BJ, Hong SW, Pyo JY. Preoperative cytologic diagnosis of Warthin-like variant of papillary thyroid carcinoma. J Pathol Transl Med. 2018; 52:105–9.

55. Kalantri SH, D’Cruze L, Barathi G, Singh BK. Warthin-like papillary carcinoma thyroid. J Cancer Res Ther. 2023; 19:1471–3.

56. Hryshchyshyn A, Bahrii A, Botsun P, Chuba V. Warthin-like variant of papillary thyroid carcinoma with lymph node metastases: a case report and review of the literature. J Med Case Rep. 2024; 18:17.
57. Chong Y, Suh S, Kim TJ, Lee EJ. Fine needle aspiration cytology of Warthin-like papillary thyroid carcinoma: a brief case report. Korean J Pathol. 2014; 48:170–3.

58. Ohashi R. Solid variant of papillary thyroid carcinoma: an underrecognized entity. Endocr J. 2020; 67:241–8.

59. Guleria P, Phulware R, Agarwal S, et al. Cytopathology of solid variant of papillary thyroid carcinoma: differential diagnoses with other thyroid tumors. Acta Cytol. 2018; 62:371–9.

60. Pinheiro SL, Miranda Afonso P, Damasio IL, Simoes-Pereira J, Nunes da Silva T, Leite V. Clinical significance of papillary thyroid carcinoma with solid/trabecular growth. Clin Endocrinol (Oxf). 2023; 99:335–41.

61. Herrera MF, Hay ID, Wu PS, et al. Hurthle cell (oxyphilic) papillary thyroid carcinoma: a variant with more aggressive biologic behavior. World J Surg. 1992; 16:669–74.
62. Lukovic J, Petrovic I, Liu Z, et al. Oncocytic papillary thyroid carcinoma and oncocytic poorly differentiated thyroid carcinoma: clinical features, uptake, and response to radioactive iodine therapy, and outcome. Front Endocrinol (Lausanne). 2021; 12:795184.

63. Ray A, Mahore SD. An oncocytic variant of papillary thyroid carcinoma mimicking as metastatic adenocarcinoma: a diagnostic challenge. Cureus. 2023; 15:e48425.

64. Patnaik N, Diwaker P, Varughese AS, Arora VK, Singh B. Cytomorphological features of oncocytic variant of papillary thyroid carcinoma with lymphocytic thyroiditis. Asian J Oncol. 2016; 2:85–7.

65. Li G, Lei J, Peng Q, et al. Lymph node metastasis characteristics of papillary thyroid carcinoma located in the isthmus: a single-center analysis. Medicine (Baltimore). 2017; 96:e7143.
66. Masui T, Adachi S, Uemura H, Kimura T, Kitahara T. Risk factors for the lateral cervical lymph node metastasis of papillary thyroid carcinoma: a clinical study. Mol Clin Oncol. 2023; 18:25.

67. Liu Y, Wang Y, Zhao K, et al. Lymph node metastasis in young and middle-aged papillary thyroid carcinoma patients: a SEER-based cohort study. BMC Cancer. 2020; 20:181.

68. Awny S, Abdallah A, Metwally IH, et al. Impact of age on central lymph nodes involvement in papillary thyroid cancer. BMC Cancer. 2024; 24:423.

69. Wang P, Dong Z, Zhao S, et al. Trends of the prevalence rate of central lymph node metastasis and multifocality in patients with low-risk papillary thyroid carcinoma after delayed thyroid surgery. Front Endocrinol (Lausanne). 2024; 15:1349272.

70. Choi JE, Bae JS, Lim DJ, Jung SL, Jung CK. Atypical histiocytoid cells and multinucleated giant cells in fine-needle aspiration cytology of the thyroid predict lymph node metastasis of papillary thyroid varcinoma. Cancers (Basel). 2019; 11:816.

71. Tseng FY, Hsiao YL, Chang TC. Cytologic features of metastatic papillary thyroid carcinoma in cervical lymph nodes. Acta Cytol. 2002; 46:1043–8.

72. Chang YC, Lo WC, Lo CY, Liao LJ. Occult papillary thyroid carcinoma initially presenting as cervical cystic lymph node metastasis: report of two cases. J Med Ultrasound. 2013; 21:92–6.

73. Ng JKM, Chan ABW, Li JJX. Colloid and pigmented histiocytes in lymph node aspirates as a clue to metastasis in patients with a history of papillary thyroid carcinoma. Diagn Cytopathol. 2024; 52:22–9.

Fig. 1.
Cytological features of classic papillary thyroid carcinoma show a monolayer sheet of cells with a syncytial-like appearance observed on liquid-based cytology (ThinPrep).
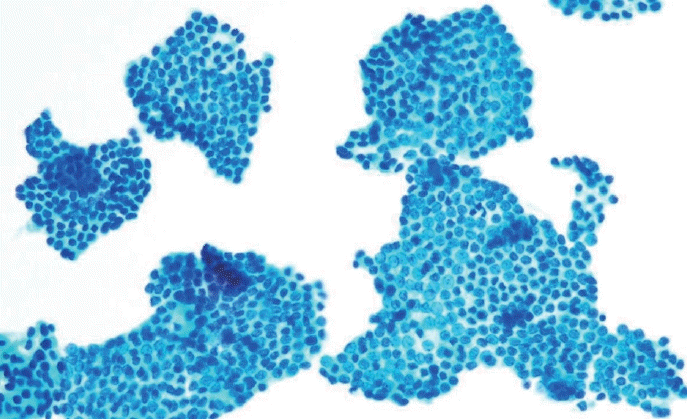
Fig. 2.
Cellular swirls (arrows), identified by flat, two-dimensional, concentrically organized aggregates of tumor cells devoid of colloid, are specific features of papillary thyroid carcinoma (A & B, ThinPrep).
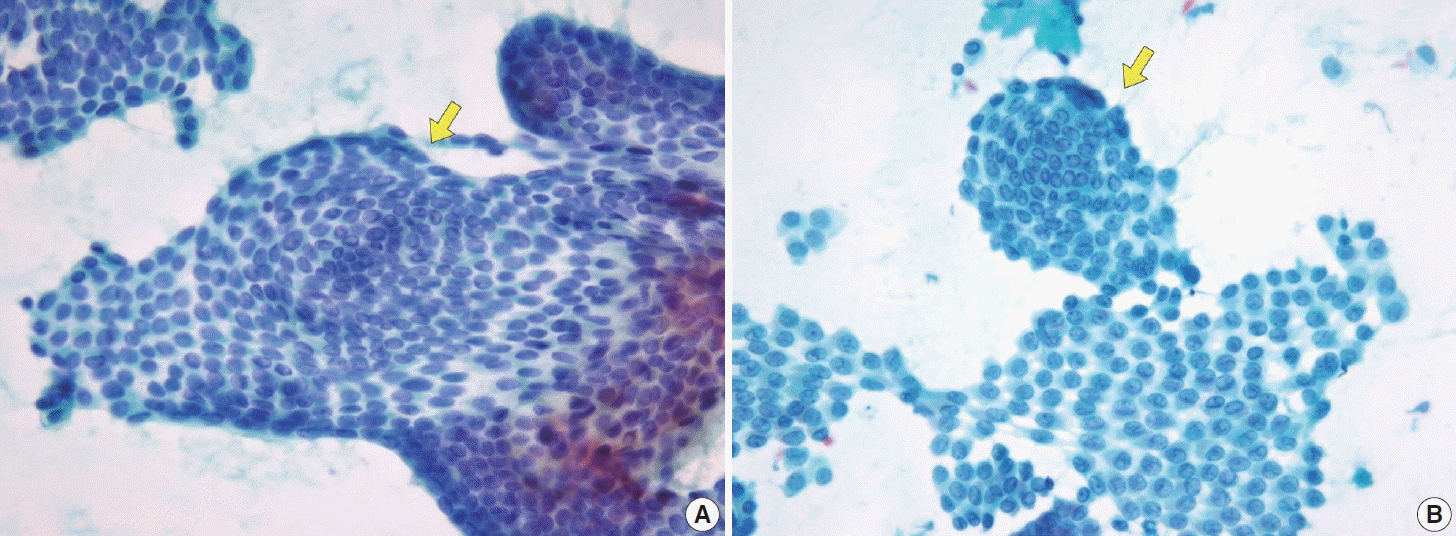
Fig. 3.
(A) Classic papillary thyroid carcinoma demonstrating typical nuclear features, including enlarged nuclei, glassy chromatin, prominent nucleoli, and intranuclear pseudoinclusions on conventional smear. (B) Liquid-based cytology smear showing a monolayer syncytial-like sheet of tumor cells with marked nuclear irregularities (ThinPrep).
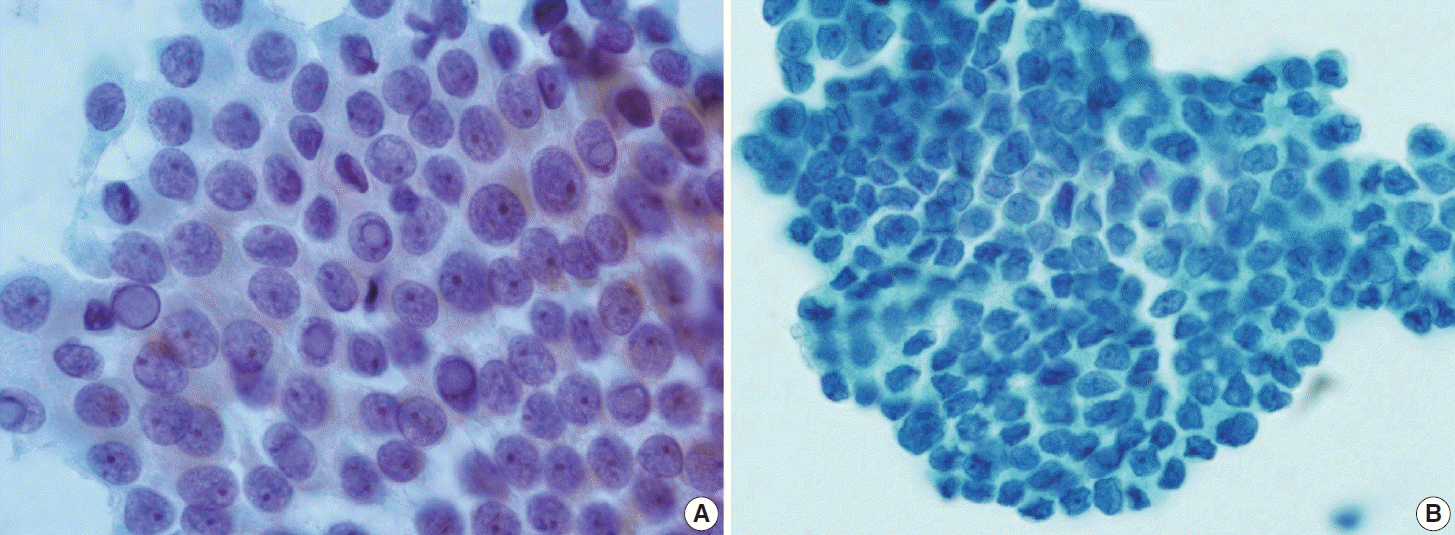
Fig. 4.
(A) Various sizes of intranuclear pseudoinclusions are visible with well-defined borders and coloration similar to the cell cytoplasm (Thin-Prep). (B) Nonspecific pseudoinclusion (arrow) lacks a clear nuclear border and may resemble bubbles, making them less distinct (ThinPrep).
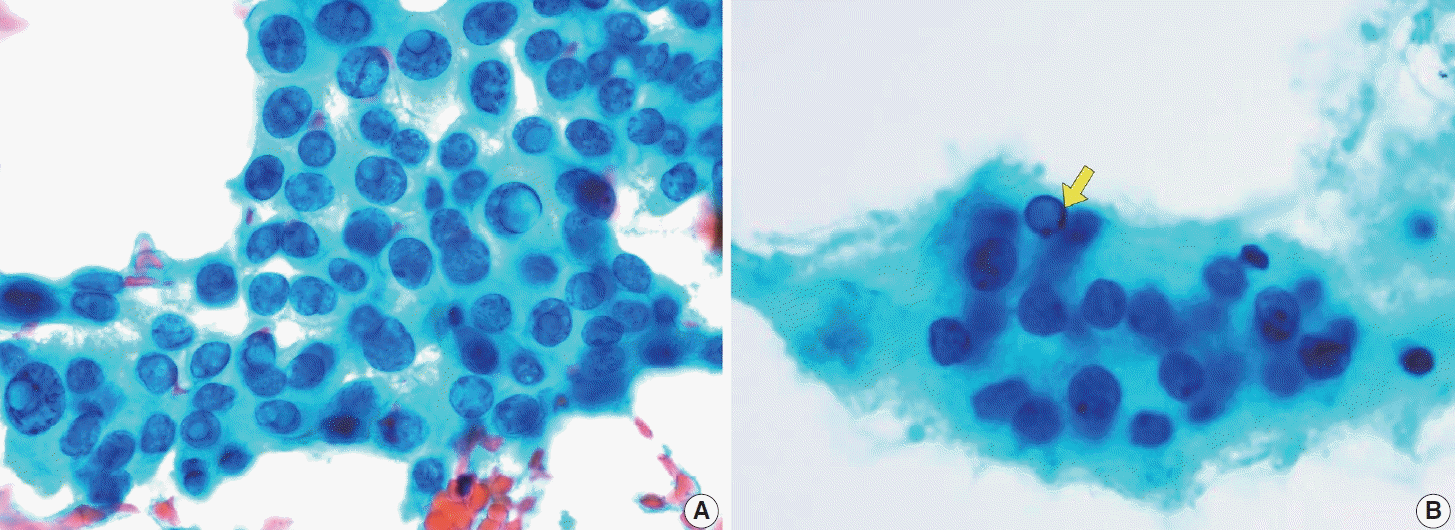
Fig. 5.
Nuclear grooves are observed as nuclear longitudinal folds. While not entirely specific, the presence of grooves in at least onethird of the tumor cell clusters, often forming branching patterns or parallel lines (insets), indicates papillary thyroid carcinoma.
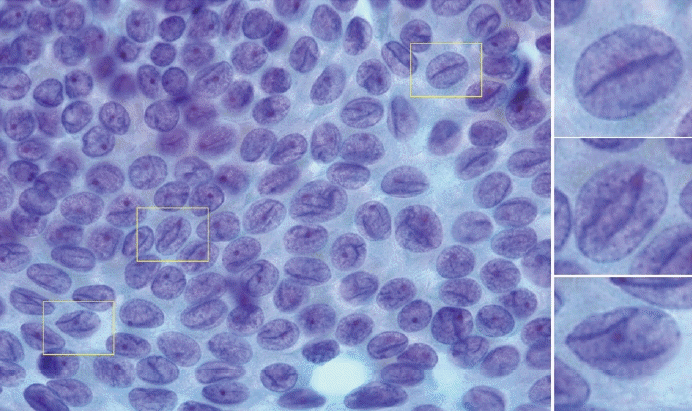
Fig. 6.
Psammoma bodies, identified as concentric calcified structures (arrows), are typically found within the papillary stalk of papillary thyroid carcinoma cells. These bodies may be absent in liquid-based cytology specimens. (A, ThinPrep; B, SurePath).
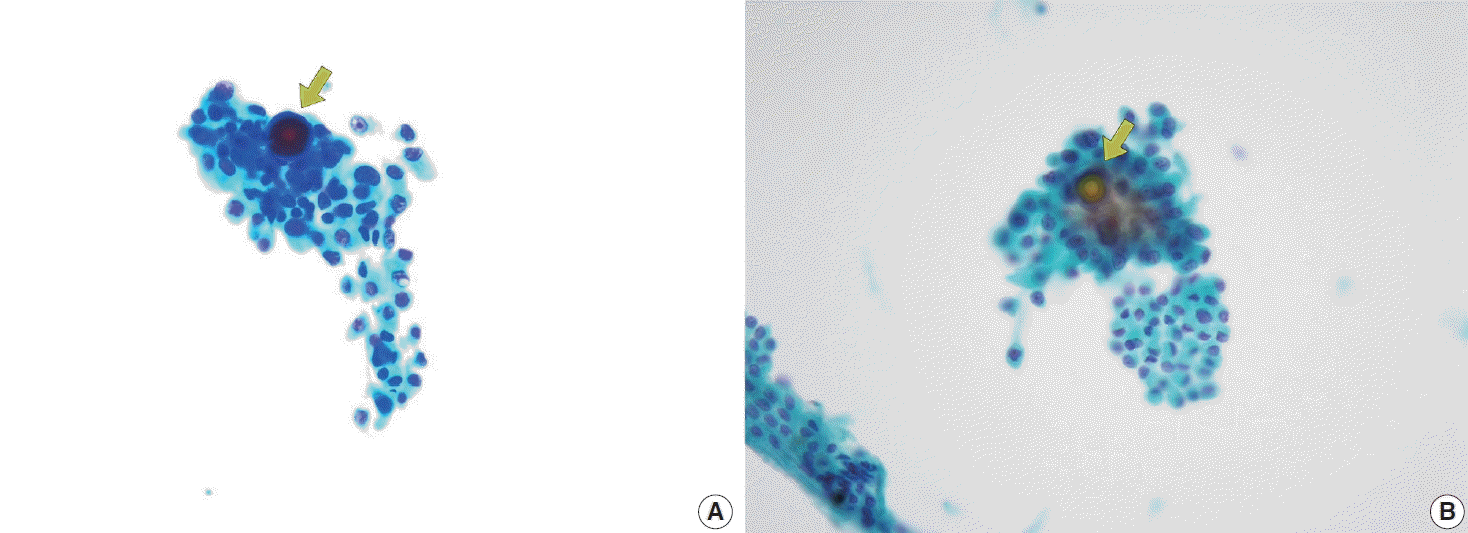
Fig. 7.
Infiltrative follicular variant of papillary thyroid carcinoma. Tumor cells are arranged in a microfollicular pattern (A) with irregular nuclear membranes, fine chromatin, and prominent nucleoli (B) (ThinPrep).
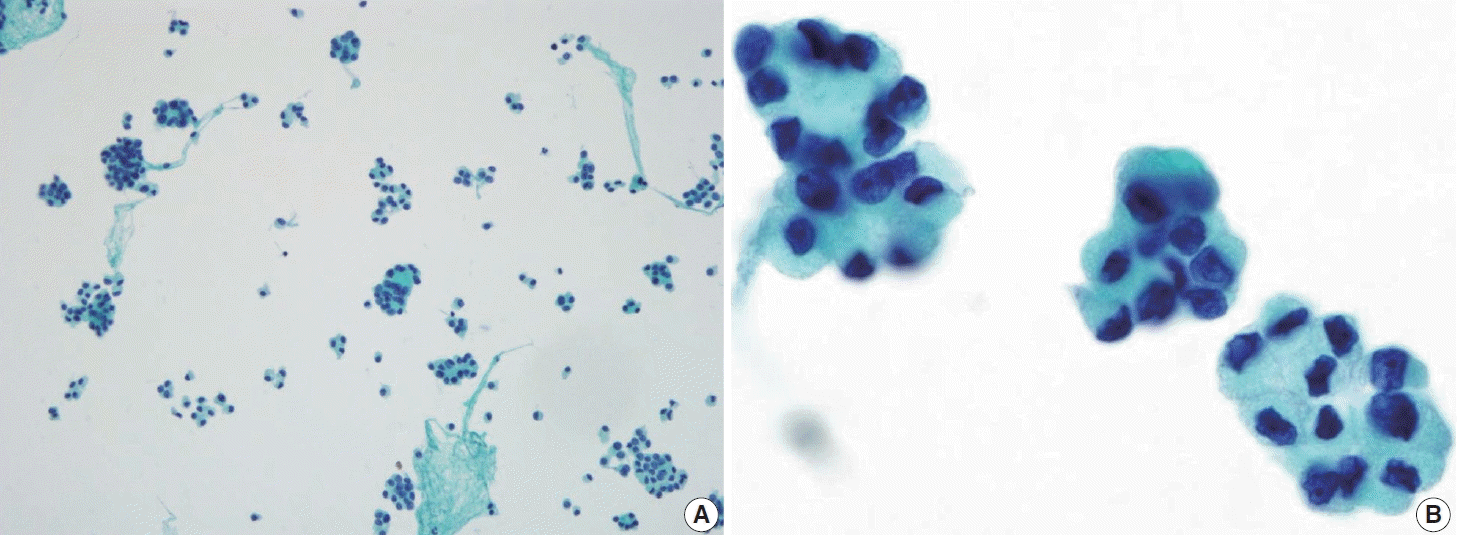
Fig. 8.
(A) Papillary thyroid carcinoma with cystic degeneration demonstrating ball-like clusters, characterized by tight, round aggregates of tumor cells with typical nuclear features such as nuclear grooves (ThinPrep). (B) Thyroid spherules, distinct from the carcinoma clusters and microfollicles, are observed as well-circumscribed round structures often surrounded by follicular cells, representing a diagnostic feature distinguishing benign follicular nodules from malignant lesions and follicular neoplasms (ThinPrep).
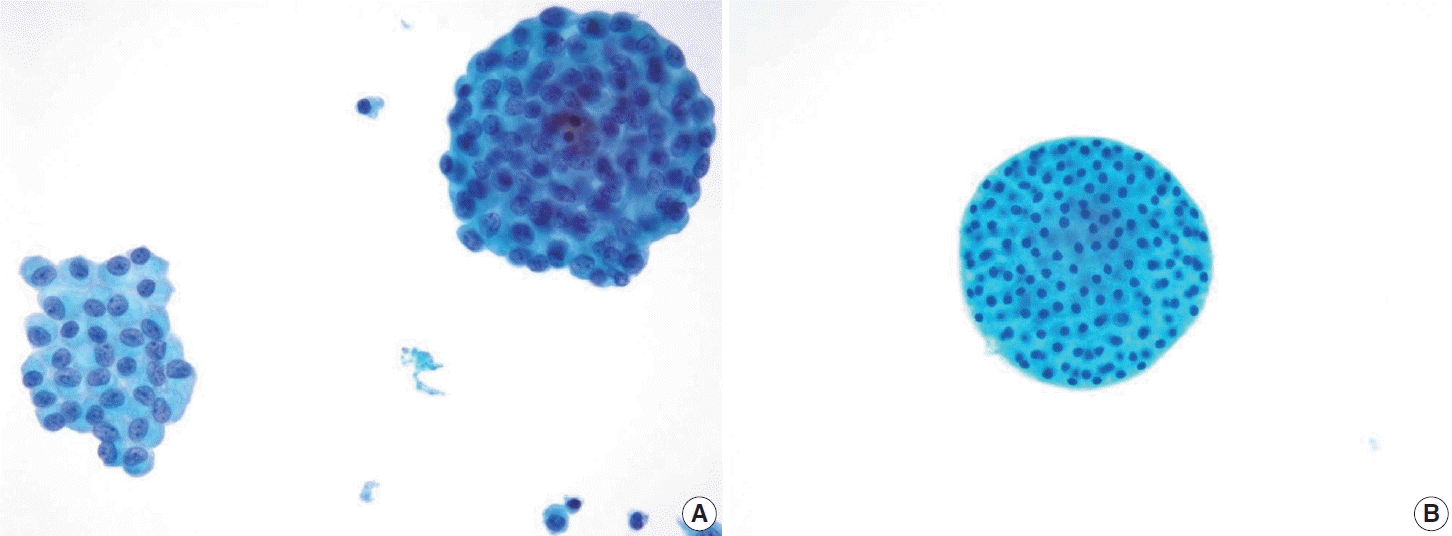
Fig. 9.
Liquid-based cytology specimen of tall cell subtype of papillary thyroid carcinoma shows parallel, elongated tumor cells with characteristic nuclear features and abundant granular cytoplasm (A, B, ThinPrep).
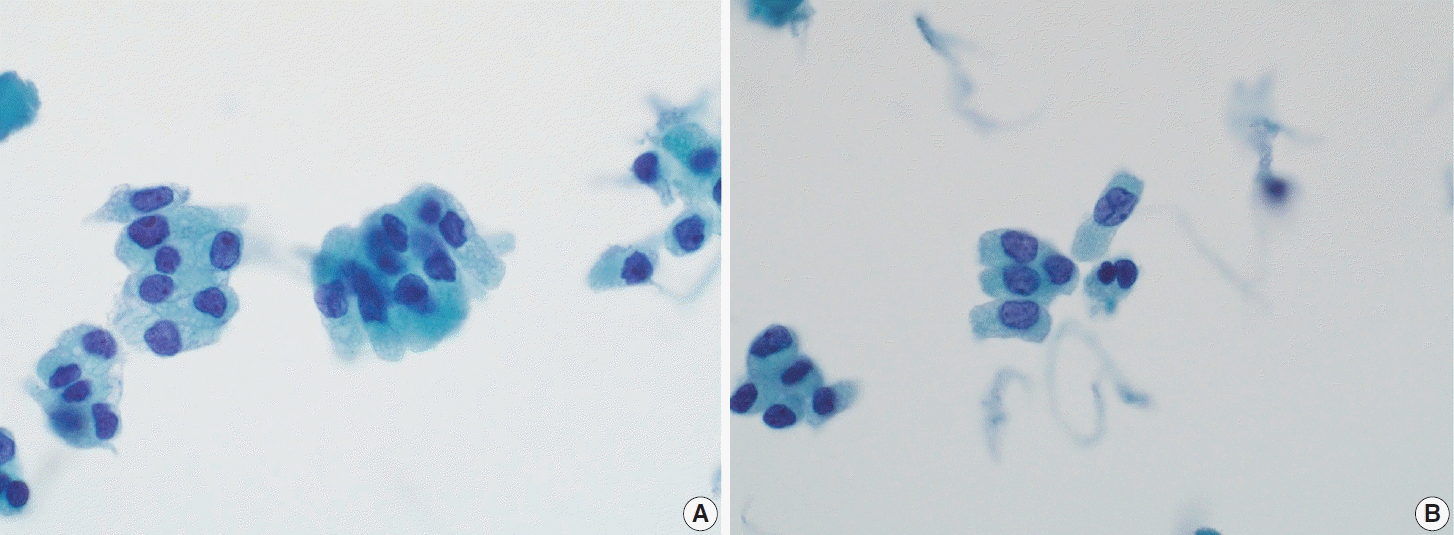
Fig. 10.
Hobnail subtype of papillary thyroid carcinoma. (A) Conventional smear shows papillary clusters of tumor cells with nuclei protruding toward the apical surface, dense chromatin, and eosinophilic cytoplasm. (B) Liquid-based cytology smear displays discohesive tumor cells with comet-like morphology (ThinPrep).
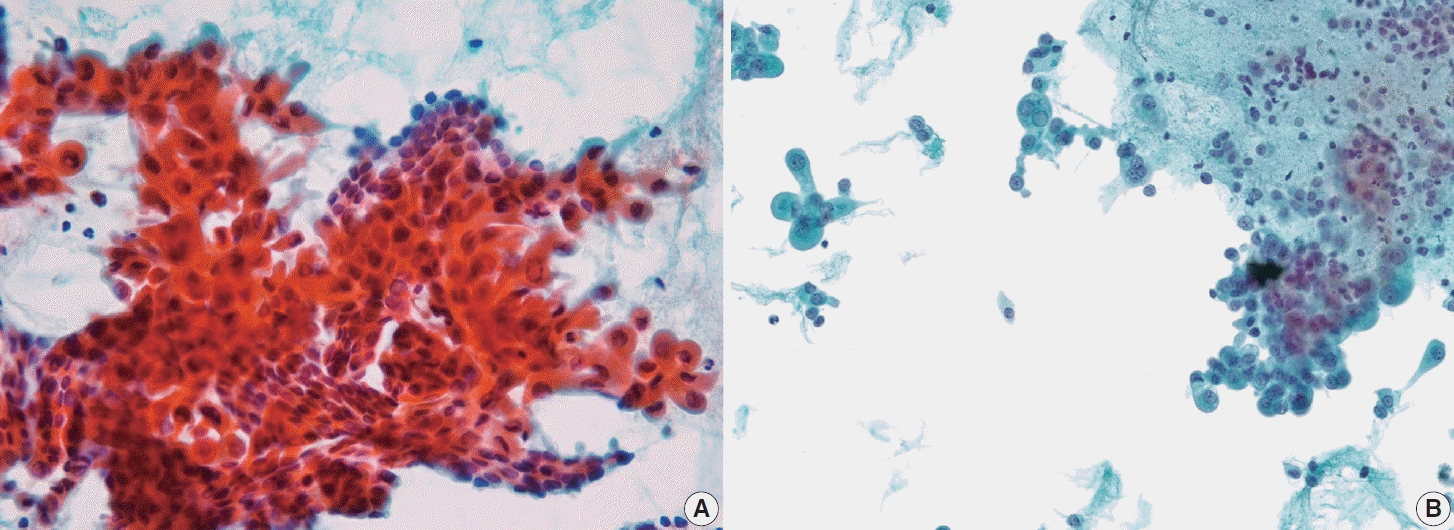
Fig. 11.
Diffuse sclerosing subtype of papillary thyroid carcinoma. (A) Cell block preparation shows tumor cells with squamous metaplasia and numerous psammoma bodies. (B) Squamous metaplasia is observed in a liquid-based cytology smear (ThinPrep).
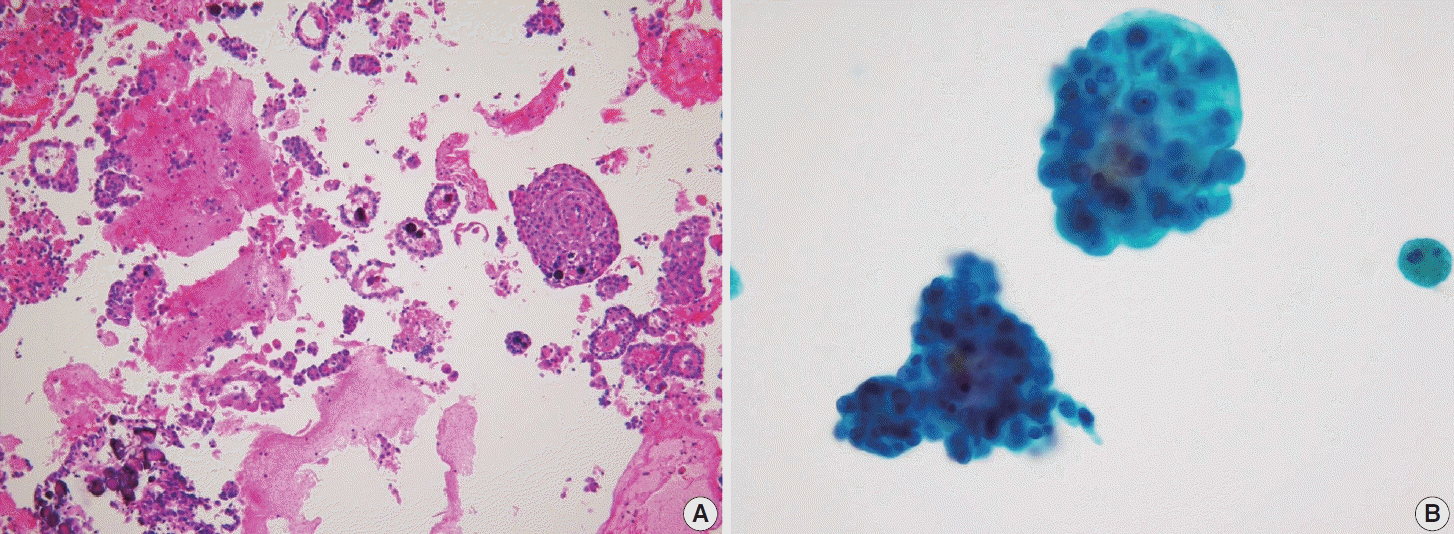
Fig. 12.
(A) Papillary thyroid carcinoma with cystic degeneration shows numerous foamy histiocytes (ThinPrep). (B) Careful examination of tumor cell clusters with characteristic nuclear features is essential for accurate diagnosis (ThinPrep).
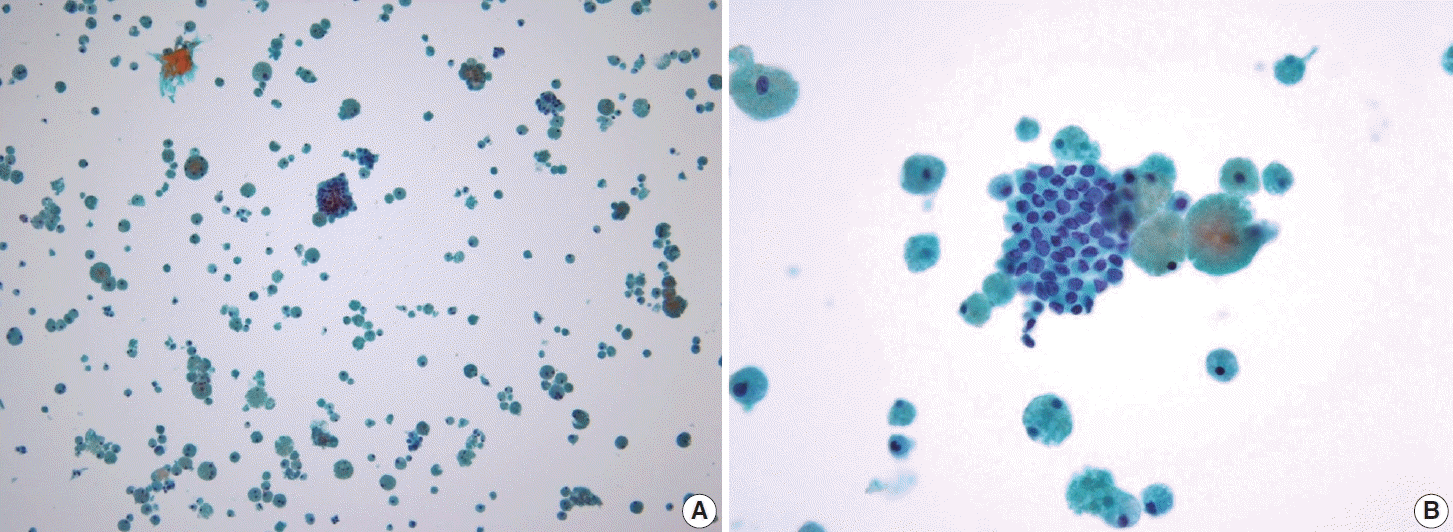
Fig. 13.
Metastasis of papillary thyroid carcinoma to the lymph node often presents with atypical histiocytoid cells resembling discohesive histiocytes with vesicular nuclei and abundant cytoplasm (A, ThinPrep), along with multinucleated giant cells (B, smear).
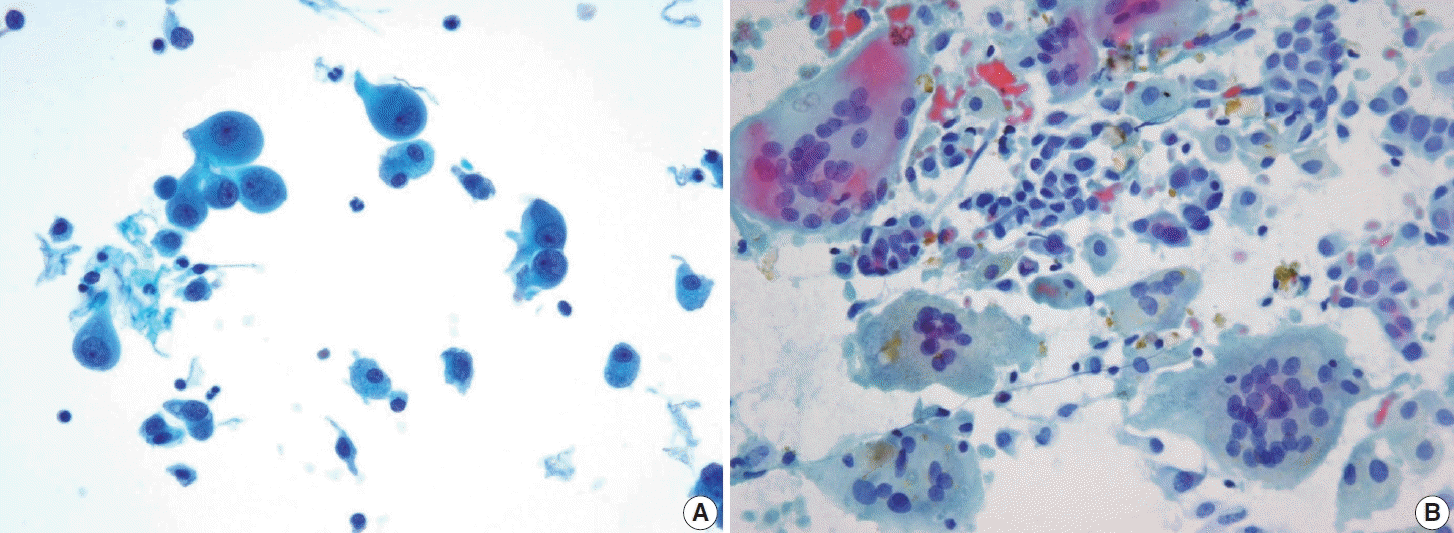
Fig. 14.
Poorly differentiated thyroid carcinoma. (A) The tumor shows high cellularity with a trabecular architecture (ThinPrep). (B) The tumor is composed of monomorphic cells characterized by a high nuclear-to-cytoplasmic ratio, coarse chromatin, and scant cytoplasm (ThinPrep).
Table 1.
Cytologic features of subtypes of PTC




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download