Abstract
Background
Methods
Results
Supplementary Information
Notes
Ethics Statement
This study was approved by the Ethics Committee of the Faculty of Medicine Universitas Indonesia – Dr. Cipto Mangunkusumo Hospital, under protocol number KET-620/UN2.F1/ETIK/PPM.00.02.2023. The requirement for informed consent was waived by the committee under protocol number ND-0313/UN2.F1.DEPT.27/PPM.00.02/2023.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: ASH, MFH, KK. Methodology: ASH, AHA, DK, MM, KK. Software: AHA. Validation: ASH and MFH. Formal analysis: MM, DK. Investigation: ASH, KK. Resources: ASH. Data curation: AA, DPR, FQ, HA, HI, HC, IW, KM, MT, NMM, NK, NA, PA, YPS, ASH. Writing— original draft preparation: ASH, AHA, DK, MM. Writing—review and editing: AA, DPR, FQ, HA, HI, HC, IW, KM, MT, NMM, NK, NA, PA, YPS, ASH, KK. Visualization: ASH, AHA. Project administration: MM. Funding acquisition: ASH. All authors have read and agreed to the published version of the manuscript.
References
Fig. 1.
Fig. 2.
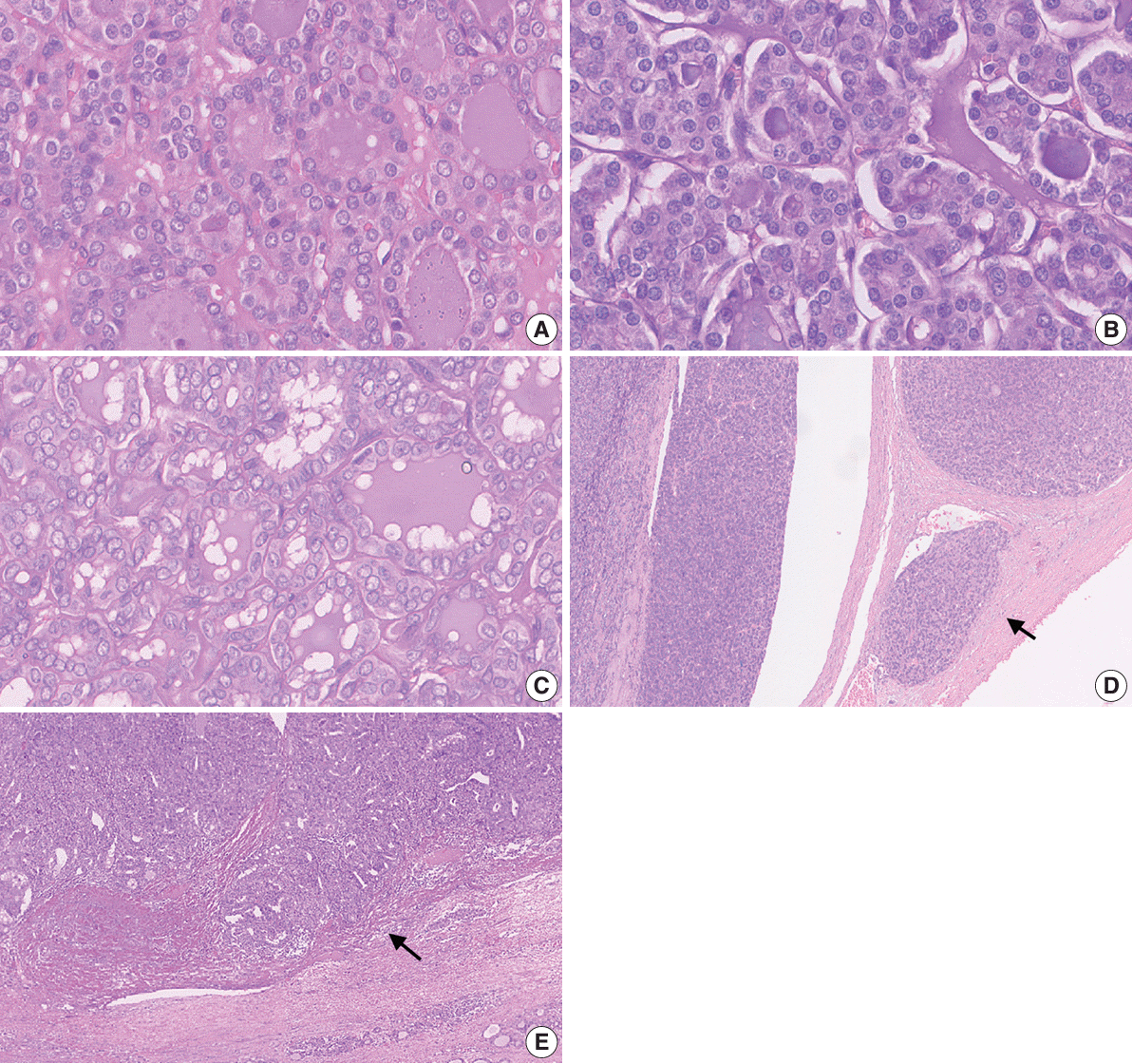
Fig. 3.
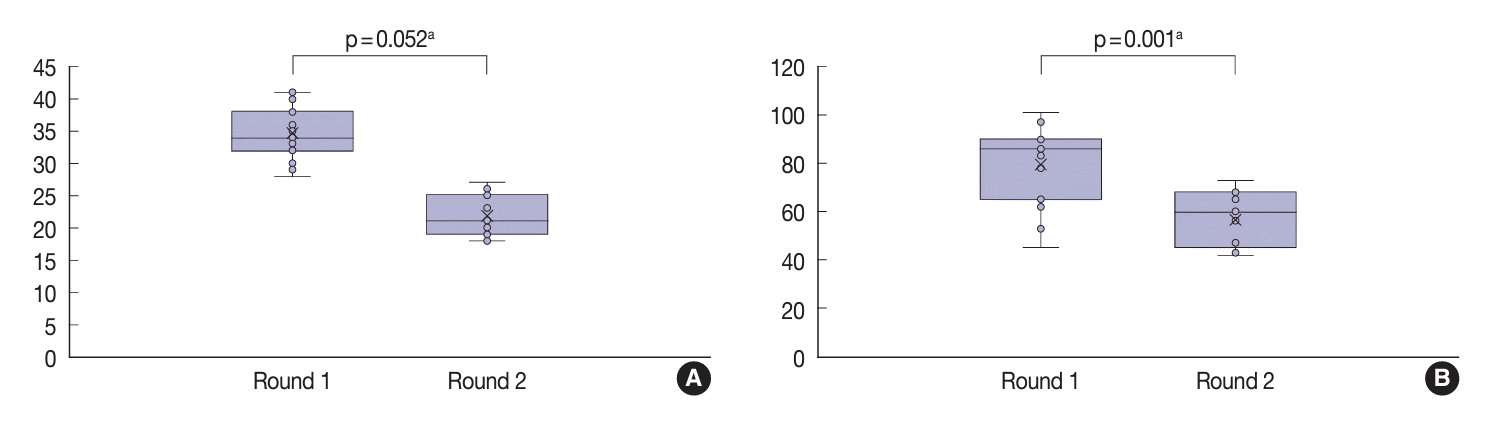
Fig. 4.
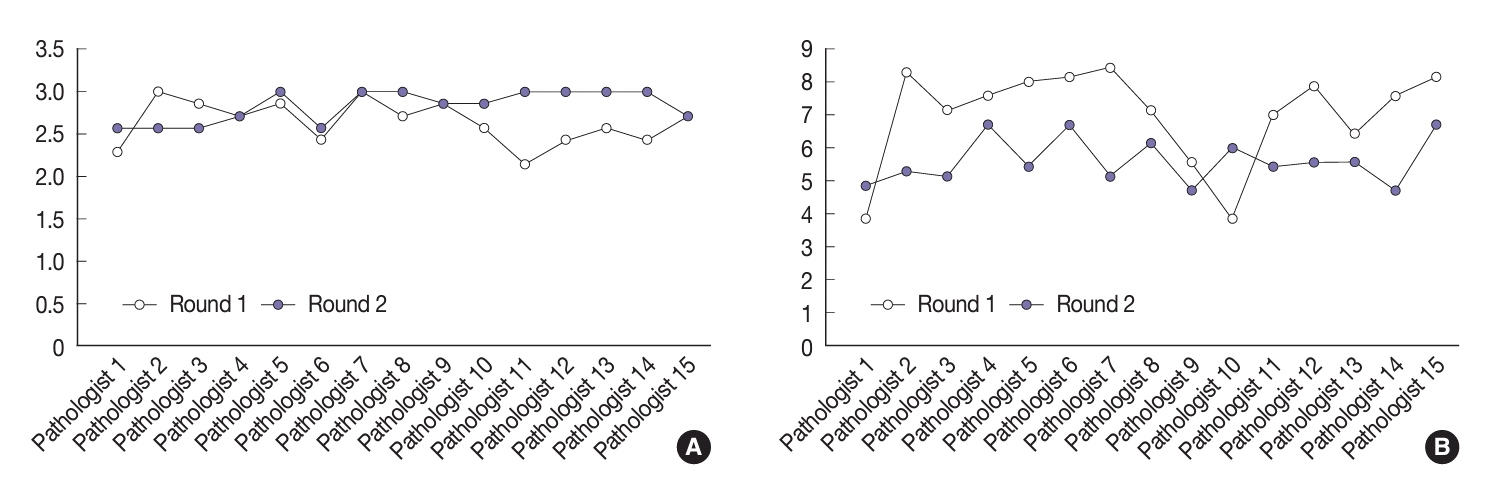
Fig. 5.
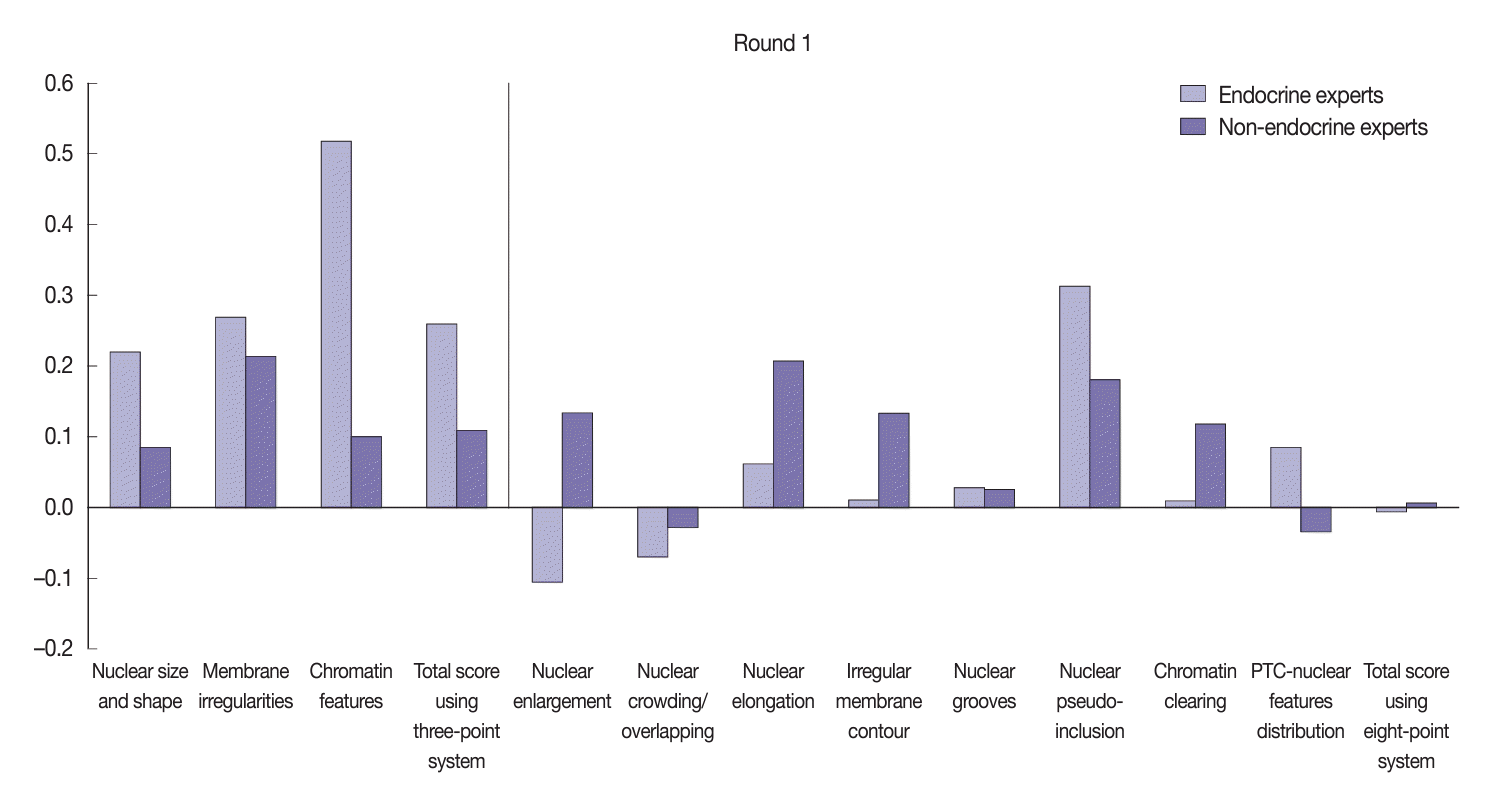
Fig. 6.
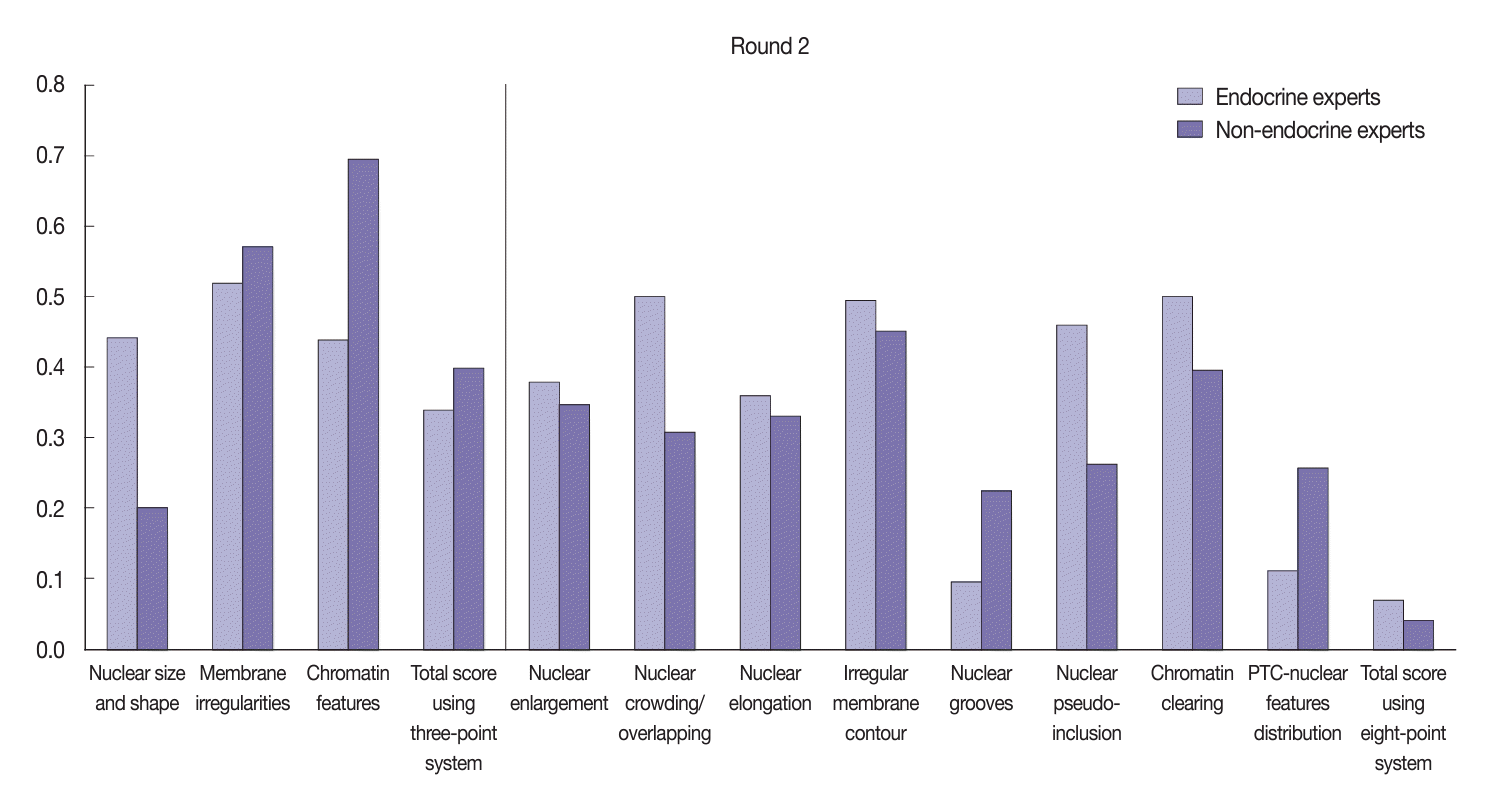
Table 1.
Table 2.
Table 3.
Table 4.
Table 5.
| Variable | Round | Mean kappa | 95% CI | p-value |
|---|---|---|---|---|
| Three-point score system | First | 0.21 | –0.57 to 0.01 | .052 |
| Second | 0.49 | |||
| Eight-point score system | First | 0.10 | –0.32 to –0.16 | .001 |
| Second | 0.34 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download