This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Disorders of amino acid metabolism fall under the category of inborn errors of metabolism that can be managed with a protein-restricted diet. However, adherence to such a diet often poses challenges, leading to low treatment engagement. Consequently, there is a pressing need for new resources to aid in dietary self-monitoring. The goal is to develop and implement “AminoApp,” an application tailored for dietary self-monitoring in patients with inborn errors of metabolism who are on a low-protein diet.
Methods
The design and development of the application adhered to the user-centered design method. This approach emphasizes active participation and collaboration between users and designers/researchers throughout all stages of product development, including requirement gathering, prototype development, and evaluation. Usability was evaluated using the System Usability Scale, which has been validated in Portuguese.
Results
The application’s features include a food diary, a food consultation area, exam records, a recipe calculator, and reports on diet composition and metabolic control. The usability test included four patients on a low-protein diet, three caregivers, and three healthcare professionals. The average usability score was 84.9, with averages of 77.5 for patients, 85.8 for caregivers, and 91.6 for professionals, indicating that the application is user-friendly.
Conclusions
AminoApp is the first application developed in Brazil designed to assist in managing inborn errors of metabolism that require a protein-restricted diet. It was found to be easy to use, and the initial results are promising. Further research is necessary to evaluate the impact of the application on metabolic control and treatment adherence.
Keywords: Inborn Errors Metabolism, Protein-Restricted Diet, Treatment Adherence and Compliance, Mobile Applications, Treatment Adherence and Compliance
I. Introduction
Inborn errors of metabolism (IEM) are genetic diseases characterized by the deficiency or absence of an enzyme or transporter that affects a specific metabolic pathway, typically presenting with multisystemic clinical symptoms [
1]. There are 1,450 known IEMs, classified into 24 different categories [
2]. This study focuses on one such category, known as disorders of amino acid metabolism. The gold standard treatment for these disorders involves a combination of a low-protein diet and the intake of protein substitutes that lack the toxic amino acid [
3]. However, adherence to this diet and monitoring adherence present significant challenges. Barriers to adherence include advancing age, limited food choices due to the poor availability of low-protein foods, limited understanding of the diet, and the difficulty of managing treatment demands [
4–
6].
As mobile health solutions become more widespread, there has been a notable increase in the use of mobile applications for health monitoring [
7]. Developing these applications locally is essential to ensure they are accessible, culturally sensitive, and linguistically appropriate. For dietary monitoring applications, it is particularly important to include regional foods and utilize chemical composition tables that are specific to the area where the application will be used. This report describes the development of a mobile application designed to help patients and caregivers monitor food intake, specifically for those required to adhere to a protein-restricted diet. Additionally, the application serves as a tool for healthcare professionals to assess their patients’ dietary intake.
II. Case Description
This case report describes the development of an application. The study protocol received approval from the local research ethics committee (Opinion No. 44023821700005327).
The design and development of the AminoApp application adhered to the user-centered design (UCD) approach. This methodology emphasizes collaboration between users and designers or researchers [
8,
9] (
Figure 1). The development process included only adult patients or caregivers.
The development followed a stepwise process:
(1) Requirement gathering: This step involved understanding the needs and desires of patients, caregivers, and professionals regarding a dietary self-monitoring application, as well as defining typical profiles of parents or families who might benefit from such an application.
(2) Development: The first version of the application was developed based on the requirements gathered and a review of similar applications already available.
(3) Evaluation: At this stage, the usability of the application was assessed using the System Usability Scale (SUS) [10], a widely recognized tool for evaluating systems and devices that has been validated in Portuguese [11]. The SUS includes 10 Likert-type questions, each scored from 1 to 5, ranging from strongly agree to strongly disagree. To calculate the composite usability score, the scores for each item are first summed. For odd-numbered items, the scale position minus 1 is used, and for even-numbered items, 5 minus the scale position is used. This sum is then multiplied by 2.5. Scores can range from 0 to 100, with scores above 68 considered above average [12]. Research indicates that the most cost-effective usability analysis typically involves 3 to 5 users per group [13].
The inclusion criteria were patients and caregivers of patients, over 18 years old, diagnosed with an amino acid metabolism disorder managed with a low-protein diet, who consented to participate. This group also included medical practitioners and nutritionists who oversee patients on such diets. The exclusion criteria ruled out pregnant women, patients who irregularly used protein substitutes, those on alternative feeding methods, and patients or caregivers with cognitive impairments that would hinder their ability to use the tool effectively. Participants were approached during a scheduled outpatient visit. Those who consented to participate signed the informed consent form and were given a week to familiarize themselves with the application. After this period, they completed the SUS scale using a standardized online form.
1. Data Source
Nutritional information—including energy, protein, carbohydrate, lipid, vitamin, and mineral content of foods—was sourced from the Brazilian Food Composition Table (TBCA) [
14]. Data concerning the amino acid composition of foods were derived from the food chemical composition tables of the United States Department of Agriculture [
15] and the Brazilian National Health Surveillance Agency (ANVISA) [
16].
2. Results
1) Requirement gathering
This stage was carried out through online focus group meetings, which included five health professionals (nutritionists), five family members of patients with tyrosinemia, phenylketonuria, propionic acidemia, and classical homocystinuria, and a representative of a patients’ association. The following requirements were established for the application:
(1) Inclusion of the following disorders of amino acid metabolism: phenylketonuria, maple syrup urine disease, homocystinuria unresponsive to pyridoxine, urea cycle disorders, tyrosinemia, propionic acidemia, methylmalonic acidemia, glutaric acidemia, and isovaleric acidemia.
(2) Food diary functionality that allows users to record meals and monitor protein intake.
(3) A food composition checker to consult regarding the nutritional composition of foods.
(4)A test results log for recording disease-specific laboratory results, with graphical visualization to assess longitudinal trends.
(5) A recipe calculation that allows users to input the ingredients of a recipe and obtain nutritional information.
(6) Generation of reports that include calculations of food records and metabolic control, which can be provided to the patient’s nutritionist to aid in dietary evaluation and prescription.
(7) Notification alerts to serve as a reminder to take protein substitute.
(8) A “Learn more” section with links to websites where users can find reliable information.
2) Development of the application
After collecting requirements from patients, the application was developed through biweekly meetings where functions were evaluated and feedback was provided. The initial version of the application was subsequently released for free on iOS, Android, and desktop platforms.
Upon first accessing the application, users are prompted to create an account and provide their date of birth, body weight, and height. Subsequently, they are directed to a screen where they can select their specific disorder and input their daily intake of certain proteins and amino acids, as recommended by their nutritionist (
Figure 2). Following this, users are taken to the home screen, which incorporates all the features identified as necessary by the focus group (
Figure 3). The application can be accessed at the following link:
https://amino-app-hcpa.vercel.app/login.
3) Application evaluation
Usability testing was conducted with four patients on a low-protein diet, three caregivers, and three healthcare professionals (
Table 1). The average scores were 77.5 for the patient group, 85.8 for the caregivers, and 91.6 for the professionals. The overall average score was 84.9, which is classified as “above average” usability (>68) (
Table 2). Consequently, the application can be deemed easy to use by all targeted user groups.
Among the 10 test items, the highest positive responses were related to ease of use (3.9), well-integrated functions (3.5), and high confidence levels (3.4). Conversely, the items that received the highest negative usability scores were the need for technical support (3.4) and the requirement for extensive learning (3.6).
III. Discussion
AminoApp is the first application developed to support the dietary management of IEMs in Brazil and the broader Portuguese-speaking community. It has been rated as user-friendly by a predominantly patient and caregiver user base, as well as by healthcare professionals specializing in IEMs. Two other applications have been developed to aid in managing low-protein diets, both of which are available outside Brazil [
17,
18]. The first, Metabolic Diet app, was launched in Canada in 2016. It features a nutrient intake calculator, a recipe calculator, and a food diary. AminoApp, in addition to these tools, provides functionalities for recording and longitudinally monitoring test results, generating reports, and setting reminders for protein substitute formula intake [
17]. The second application, PKU Bite, was created in the United Kingdom. As indicated by its name, PKU Bite is specifically designed for individuals with phenylketonuria (PKU). In contrast, AminoApp caters to a broader range of IEMs that require a low-protein diet. PKU Bite assists users in selecting appropriate foods, interpreting food labels, and calculating protein intake [
18].
By using the UCD method for application development, we gathered valuable feedback from users. This allowed us to incorporate innovative features, such as the capability to record and visually track test results over time, alongside food reports that aid nutritionists in assessing diets. Moreover, all features of AminoApp align with the preferences caregivers expressed in a recent study [
19].
It is imperative to note that the patient group recorded the lowest average score on the application usability test, at 77.5. However, this score still surpasses the overall average of 68. This discrepancy may be linked to findings from a study indicating that as patients mature and gain a better understanding of their dietary needs, their motivation and willingness to adhere to the diet diminish. This decline in adherence is often due to the increased responsibilities associated with adulthood, such as work and childcare [
20].
The results of this study are promising. We anticipate that using the application will enhance patient engagement and commitment to their treatment. This should lead to improved dietary adherence and better metabolic control, potentially preventing long-term cognitive damage.
One limitation of the application is its lack of accessibility tools for visually impaired users. Additionally, the application has not been evaluated for its actual impact on dietary and metabolic control, though we plan to conduct this assessment in the near future.
Acknowledgments
The authors acknowledge the Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS) - Post- Doctoral Fellowship - ARD (n. 21/2551-0000625-0) for providing funding for the project. The authors also thank Simone Arede, Center for Innovation and Technology Transfer (Nitt) and Financiamento e Incentivo à Pesquisa (FIPE) do Hospital de Clínicas de Porto Alegre, the Coordination for the Improvement of Higher Education Personnel (CAPES), Recordati Rare Diseases, and CMW Saúde for their support. We are immensely grateful to all the patients and their families for their collaboration.
References
2. Ferreira CR, Rahman S, Keller M, Zschocke J; ICIMD Advisory Group. An international classification of inherited metabolic disorders (ICIMD). J Inherit Metab Dis. 2021; 44(1):164–77.
https://doi.org/10.1002/jimd.12348.

3. Agana M, Frueh J, Kamboj M, Patel DR, Kanungo S. Common metabolic disorder (inborn errors of metabolism) concerns in primary care practice. Ann Transl Med. 2018; 6(24):469.
https://doi.org/10.21037/atm.2018.12.34.

4. MaCdonald A, van Rijn M, Feillet F, Lund AM, Bernstein L, Bosch AM, et al. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann Nutr Metab. 2012; 61(4):289–95.
https://doi.org/10.1159/000342256.

5. Teruya KI, Remor E, Schwartz IV. Factors that increase risk for poor adherence to phenylketonuria treatment in Brazilian patients. Am J Med Genet A. 2021; 185(7):1991–2002.
https://doi.org/10.1002/ajmg.a.62195.

6. Vieira TA, Nalin T, Krug BC, Bittar CM, Netto CB, Schwartz IVD. Adherence to treatment of phenylketon-uria: a study in southern Brazilian patients. J Inborn Errors Metab Screen. 2015; 3:2326409815579861.
https://doi.org/10.1177/2326409815579861.

7. Perez-Jover V, Sala-Gonzalez M, Guilabert M, Mira JJ. Mobile apps for increasing treatment adherence: systematic review. J Med Internet Res. 2019; 21(6):e12505. DOI:
10.2196/12505.

9. McCurdie T, Taneva S, Casselman M, Yeung M, Mc-Daniel C, Ho W, et al. mHealth consumer apps: the case for user-centered design. Biomed Instrum Technol. 2012. Suppl. 49–56.
https://doi.org/10.2345/0899-8205-46.s2.49.

10. Jordan PW, Thomas B, McClelland IL, Weerdmeester B. Usability evaluation in industry. Boca Raton (FL): CRC Press;1996.
11. Tenorio JM, Cohrs FM, Sdepanian VL, Pisa IT, Marin HD. Desenvolvimento e Avaliacao de um Protocolo Eletronico para Atendimento e Monitoramento do Paciente com Doenca Celiaca. Rev Informatica Teorica e Apl. 2011; 17(2):210–20.
https://doi.org/10.22456/2175-2745.12119.

14. Brazilian food composition table (TBCA) [Internet]. Sao Paulo, Brazil: Universidade de Sao Paulo, Food Research Center;2023. [cited at 2024 Jul 16]. Available from:
https://www.tbca.net.br/.
15. United States Department of Agriculture. FoodData Central search results [Internet]. Washington (DC): United States Department of Agriculture;2018. [cited at 2024 Jul 16]. Available from:
https://fdc.nal.usda.gov/fdc-app.html#/.
18. Evans S, Ashmore C, Daly A, Dhadwar P, Syed A, Lecocq O, et al. Efficacy of a new low-protein multimedia diet app for PKU. Nutrients. 2022; 14(11):2182.
https://doi.org/10.3390/nu14112182.

19. Lim JY, Ali NM, Rajikan R, Amit N, Hamid HA, Leong HY, et al. Need analysis of a dietary application among caregivers of patients with disorders of amino acid metabolism (AAMDs): a mixed-method approach. Int J Med Inform. 2023; 177:105120.
https://doi.org/10.1016/j.ijmedinf.2023.105120.

20. Cazzorla C, Bensi G, Biasucci G, Leuzzi V, Manti F, Musumeci A, et al. Living with phenylketonuria in adulthood: the PKU attitude study. Mol Genet Metab Rep. 2018; 16:39–45.
https://doi.org/10.1016/j.ymgmr.2018.06.007.

Figure 1
User-centered design process. Adapted from McCurdie et al. mHealth consumer apps: the case for user-centered design. Biomed Instrum Technol 2012;Suppl: 49–56 [
9].
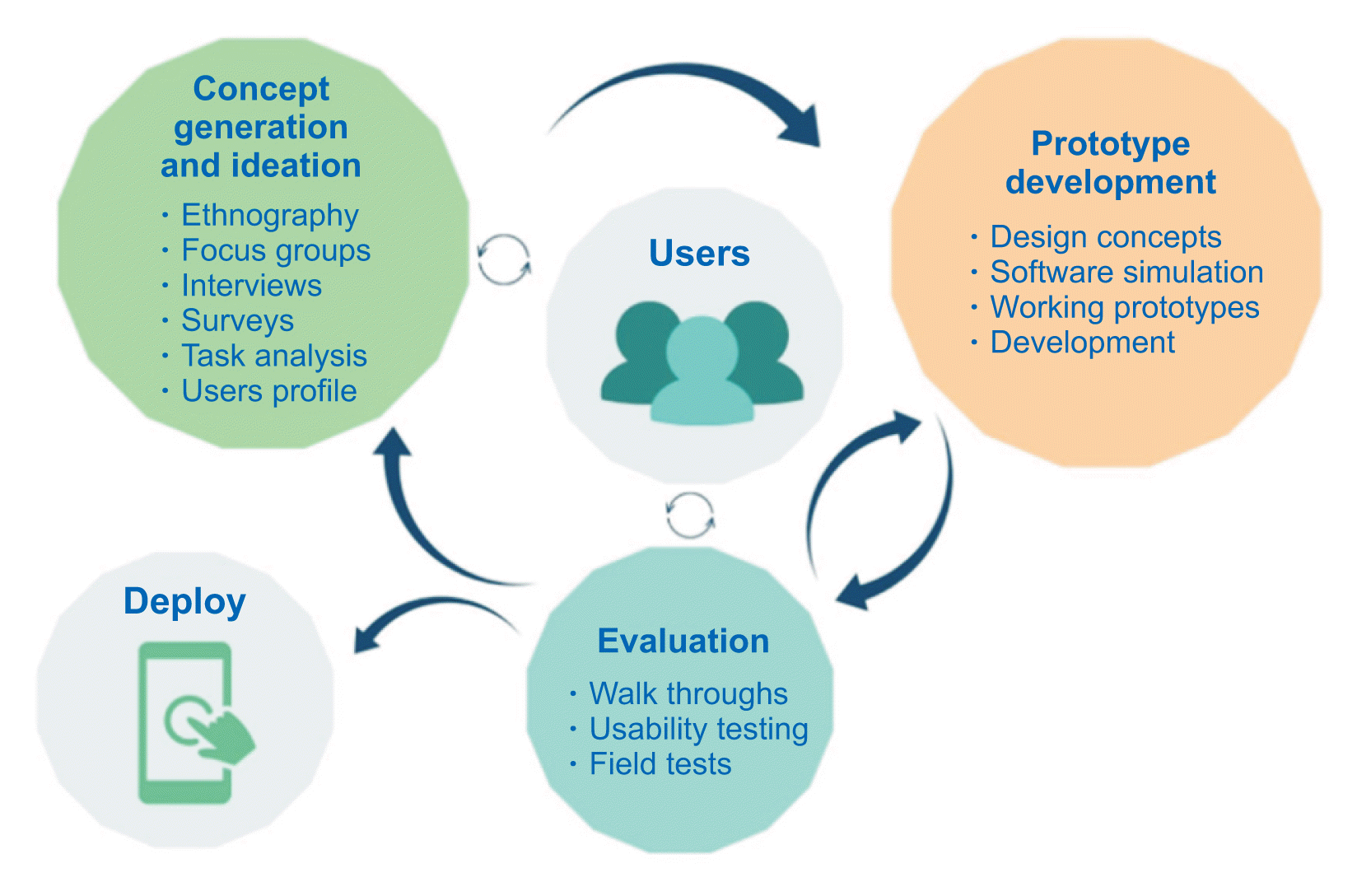
Figure 2
AminoApp account creation flow: (A) create an account, (B) choose the controlled nutrients, and (C) select the disorder.
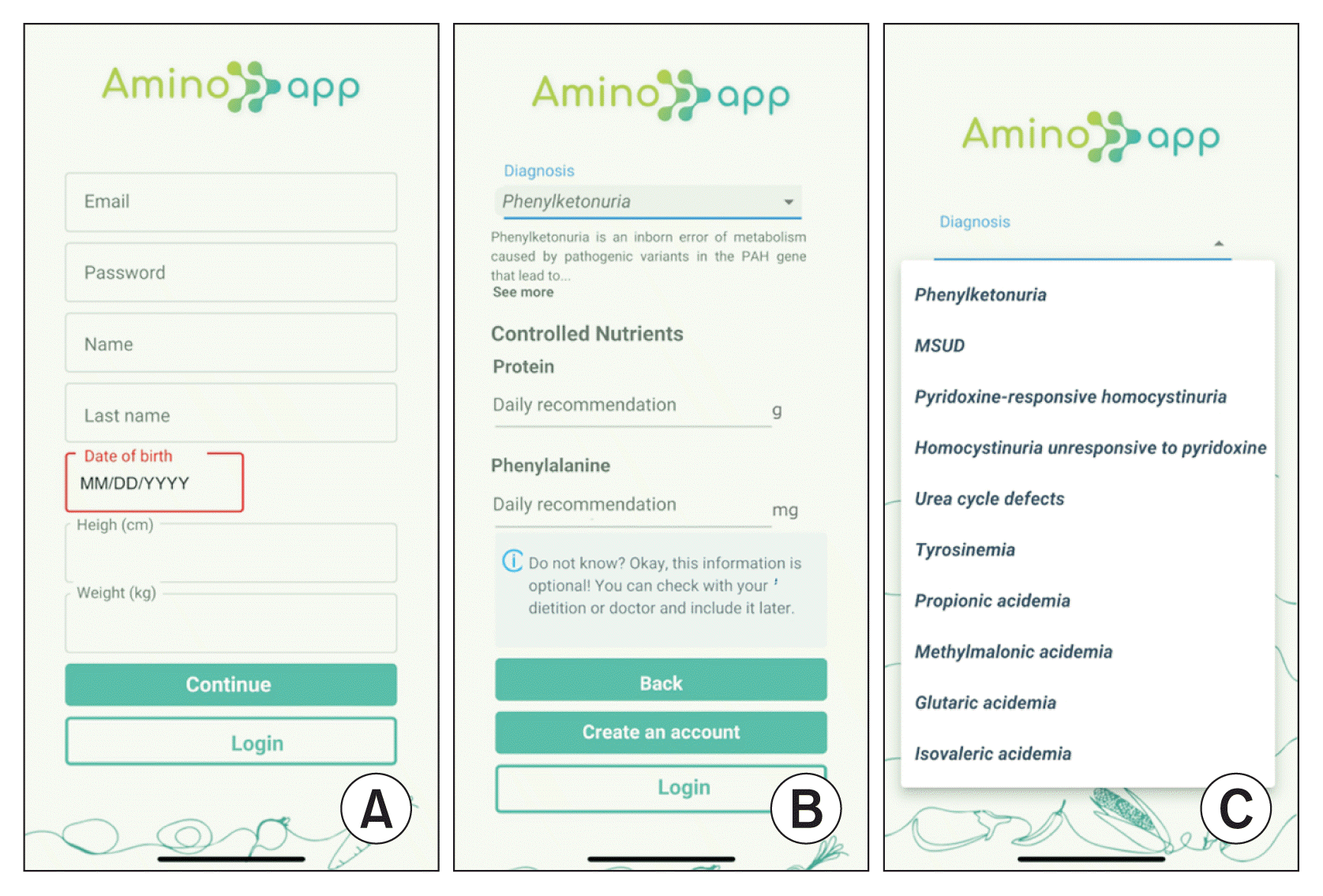
Figure 3
AminoApp screens: (A) home screen, (B) food diary, (C) food composition checker, (D) test results log, (E) recipe calculation, (F) reports, (G) reminder formula, and (H) “Learn more” links.
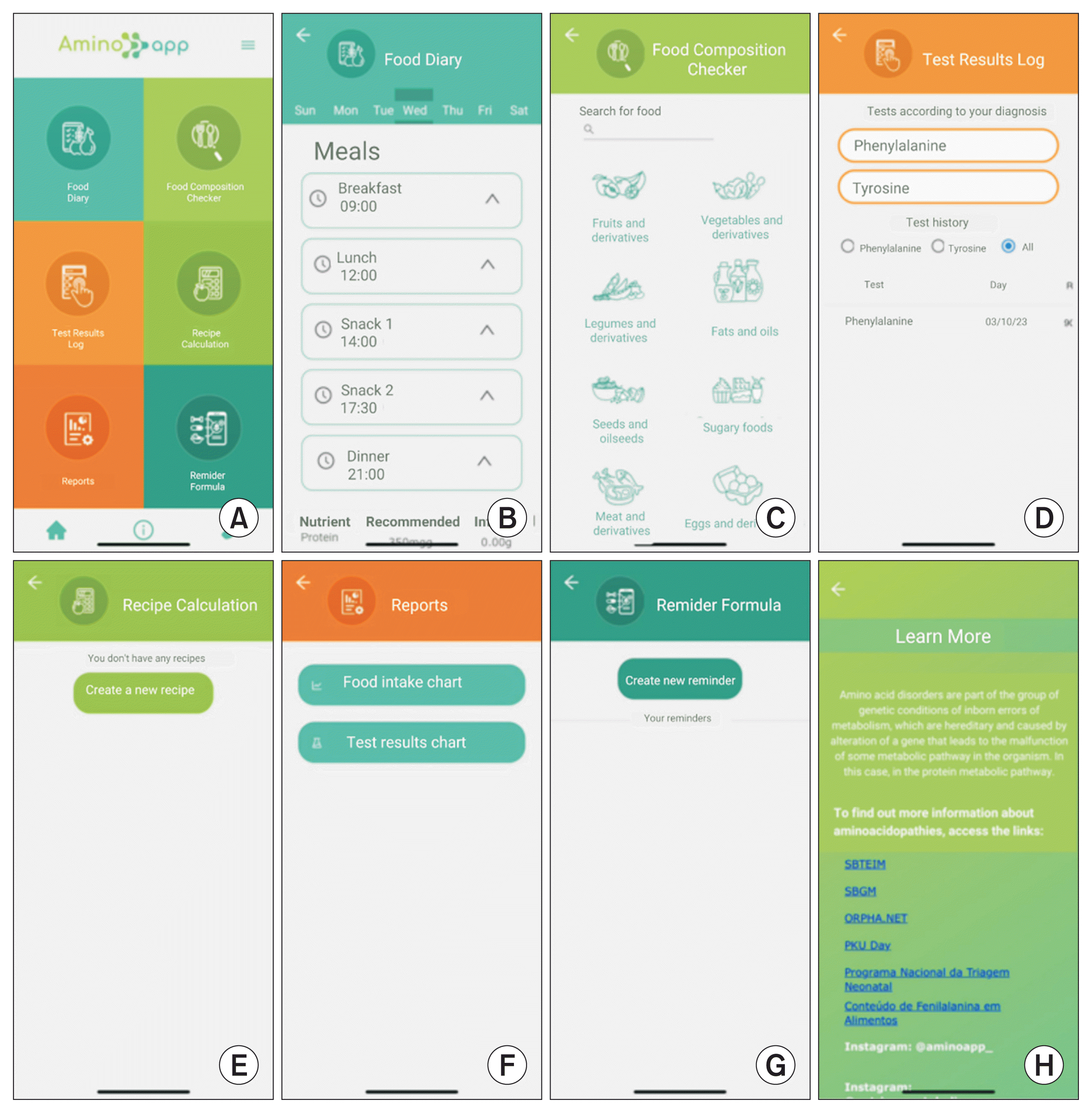
Table 1
Participant characteristics
|
Characteristic |
Patients |
Caregivers |
Professionals |
|
|
|
|
1 |
2 |
3 |
4 |
1 |
2 |
3 |
1 |
2 |
3 |
|
Disease |
Homocystinuria |
Phenylketonuria |
Argininosuccinic aciduria |
Phenylketonuria |
Propionic acidemia |
Maple syrup urine disease |
Phenylketonuria |
- |
- |
- |
|
|
Sex |
Female |
Male |
Female |
Female |
Female |
Female |
Female |
Female |
Male |
Female |
|
|
Age (yr) |
41 |
34 |
25 |
27 |
34 |
43 |
36 |
59 |
38 |
60 |
|
|
Specialty |
- |
- |
- |
- |
- |
- |
- |
Dietitian |
Doctor |
Dietitian |
Table 2
System Usability Scale (SUS) scores
|
Patients |
Caregivers |
Professionals |
Average score for each item |
|
|
|
|
HCU |
PKU |
ASA |
PKU |
PA |
MSUD |
PKU |
Dietitian |
Doctor |
Dietitian |
|
Positive questions |
|
Would use frequently |
2 |
1 |
4 |
2 |
4 |
4 |
4 |
2 |
4 |
4 |
3.3 |
|
Easiness to use |
4 |
3 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
3.9 |
|
Functions well integrated |
3 |
3 |
3 |
4 |
4 |
4 |
2 |
4 |
4 |
4 |
3.5 |
|
Easiness to learn |
4 |
2 |
1 |
3 |
2 |
4 |
4 |
3 |
3 |
3 |
2.9 |
|
Felt very confident |
3 |
3 |
4 |
4 |
3 |
3 |
3 |
4 |
4 |
4 |
3.4 |
|
|
Negative questions |
|
Unnecessarily complex |
4 |
4 |
1 |
4 |
4 |
4 |
3 |
4 |
1 |
4 |
3.3 |
|
Need technical support |
4 |
3 |
0 |
4 |
3 |
4 |
4 |
4 |
4 |
4 |
3.4 |
|
Too much inconsistency |
4 |
3 |
1 |
4 |
2 |
4 |
2 |
4 |
4 |
4 |
3.2 |
|
Very cumbersome |
3 |
3 |
3 |
4 |
3 |
4 |
3 |
4 |
1 |
4 |
3.2 |
|
Needed to learn a lot |
3 |
3 |
4 |
4 |
3 |
3 |
4 |
4 |
4 |
4 |
3.6 |
|
|
SUS score |
85 |
70 |
62.5 |
92.5 |
80 |
95 |
82.5 |
97.5 |
82.5 |
95 |
|
|
|
Mean SUS score per group |
|
77.5 |
|
|
85.8 |
|
|
91.6 |
|
|
|
|
Total mean SUS score |
|
|
|
|
84.9 |
|
|
|
|
|




 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download