Abstract
Objectives
This study was performed to examine the content of decision-making support and patient responses, as documented in the nursing records of individuals with cancer. These patients had received outpatient treatment at hospitals that met government requirements for providing specialized cancer care.
Methods
Nursing records from the electronic medical record system (in the subjective, objective, assessment, and plan [SOAP] format), along with data from interviews, were extracted for patients receiving outpatient care at the Department of Internal Medicine and Palliative Care and the Department of Breast Oncology. Data analysis involved simple tabulation and text mining, utilizing KH Coder version 3.beta.07d.
Results
The study included 42 patients from palliative care internal medicine and 60 from breast oncology, with mean ages of 70.5 ± 12.2 and 55.8 ± 12.2 years, respectively. Decisions most frequently regarded palliative care unit admission (25 cases) and genetic testing (24 cases). The assessment category covered keywords including (1) “pain,” “treatment,” “future,” “recuperation,” and “home,” as terms related to palliative care and internal medicine, as well as (2) “treatment,” “relief,” and “genetics” as terms related to breast oncology. The plan category incorporated keywords such as (1) “treatment,” “relaxation,” and “visit” and (2) “explanation,” “confirmation,” and “conveyance.”
Conclusions
Nurses appear crucial in evaluating patients’ symptoms and treatment paths during the decision-making support process, helping them make informed choices about future treatments, care settings, and genetic testing. However, when patients cannot make a decision solely based on the information provided, clinicians must address complex psychological concepts such as disease progression and the potential genetic impact on their children. Further detailed observational studies of nurses’ responses to patients’ psychological reactions are warranted.
In 2020, the adoption rate of electronic medical records (EMRs) in general hospitals in Japan reached 57.2%. This rate was 91.2% for hospitals with 400 or more beds, demonstrating substantial progress in EMR implementation, especially among larger healthcare institutions [1]. However, electronic health record (EHR) databases are primarily designed for administrative purposes and may not be appropriately structured for clinical research. In Japan, clinical research often utilizes databases such as the Japan Medical Data Center, the Diagnosis Procedure Combination database (Ministry of Health, Labor and Welfare), the Medical Data Vision database, and the National Database. Consequently, relatively few studies have leveraged EMR or EHR data from single or multiple hospitals with smaller patient populations [2].
In leveraging EHRs, EMR patient data must be integrated with existing disease registries to support critical components and ensure optimal health outcomes. However, this carries several challenges, including issues with data standardization [3], difficulty measuring data quality [4], and the absence of a standardized approach for assessing EHR data quality [5]. One study identified factors either promoting (63%; 147 of 232) or hindering (37%; 85 of 232) the adoption of EMRs [6]. In cancer care, the use of EHRs to complement cancer registry data can help identify health behaviors, clinical characteristics, and other factors that may influence patient prognosis [7]. Notably, analysis of EMR data alone tends to overestimate survival time compared to analyses that combine EMR information with cancer registry data [8]. Therefore, when repurposing EMR data, one must consider the potential for false positives or missing cancer cases, underscoring the need for analysis by appropriately trained experts [9]. Furthermore, bias may complicate the use of EHRs to provide information for cancer surveillance [10]. EMRs in cancer care contain information on diagnosis, treatment, and decision-making about treatment, place of care, and genetic testing. This information is vital for evaluating support to improve the quality of life among patients undergoing cancer treatment. However, no survey reports have examined actual descriptions associated with decision-making support.
EHRs have gained prominence as valuable sources of real-world data (RWD). Studies have reported the use of natural language processing (NLP) and machine learning (ML) techniques in analyzing EHRs, with minimal variance (<8%) observed in the results across different data curation methods [11]. The patterns discerned in EHR data through ML can be extrapolated to real-world settings. However, these techniques often reflect the specific structures of local EHR systems and may not be generalizable to other systems [12]. Additionally, EHR data are frequently stored in an unstructured format, including a variety of documents such as clinical notes, surgical records, and prescriptions. To uncover meaningful relationships among variables that could influence patient outcomes, these data must be transformed into a structured, computable format [13].
NLP and ML techniques are increasingly used to process patient-reported outcome (PRO) data. Several algorithms have been identified, underscoring the potential of NLP/ML technologies [14]. Furthermore, various ML and deep learning techniques have been employed in named entity recognition for the International Classification of Diseases, 9th Revision; analysis of clinical records; and research into mental health disorders [15]. NLP techniques can aid in converting vast amounts of unstructured PRO data found in EHRs into actionable clinical insights for patients with cancer [16]. In this context, we focused on the palliative care and breast oncology departments, where patients with cancer frequently face complex decisions. Our aim was to use nursing records to clarify the content regarding decision support. Such support from nurses involves assisting patients in improving the quality of their decisions related to treatment options, genetic testing, and care approaches for cancer.
In this study, we analyzed nursing records documented in the EMRs of hospitals that met government requirements for specialized cancer care provision. The nursing records consisted of EMR entries completed by nurses, which included information on decision-making support for patients with cancer under the management of palliative care and breast cancer departments, as well as details from support interviews.
Records from oncology nurse specialists (OCNSs) regarding decision-making support were obtained. We extracted nursing records from the EMR system (termed NR1) and a separate record form that detailed the decision-making situation (called NR2). These records were then matched by date to integrate the data. From NR1, we obtained information including patient age, sex, diagnosis, and details of the nursing care related to decision-making. The nursing records were organized according to the subjective (S), objective (O), assessment (A), and plan (P) or SOAP format, as they were entered in a problem-based narrative progress record system. NR2 provided information on the content of the decision, patient and family reactions (specifically, changes observed after receiving decision support from an OCNS), the presence or absence of companions, and individuals who influenced the decision-making process. This information was accessed by the researcher after the data had been anonymized—that is, once patient names and other proper nouns had been removed—by the designated staff member at the medical institution.
KH Coder version 3.beta.07d was employed to analyze NR1, utilizing co-occurrence networks and multidimensional scaling methods. NR2 data were simply tabulated, and the content was analyzed.
This study was approved by the Institutional Review Board of the Research Ethics Committee, College of Nursing Art and Science and Research Institute of Nursing Care for People and Community, University of Hyogo, Japan (Approval no. 2022F03, approval date: September 22, 2022). The authors adhered to the principles outlined in the Declaration of Helsinki for all human experimental investigations. Informed consent was acquired through an opt-out option provided on the website and the outpatient bulletin board.
The decision-making support provided by the OCNSs (NR1) and a record form that detailed the decision-making situation (NR2) were obtained and analyzed.
Overall, 42 patients were managed in the palliative care department, and 60 patients were treated in the breast cancer department (Table 1). The most frequent decision in the palliative care unit (PCU) involved hospitalization (25 cases), while that in the breast oncology department related to genetic testing (24 cases) (Figure 1).
The Japanese text in the figures was not analyzed in English. The original figures in this study are presented in Japanese; however, they have been translated into English. NR1 was analyzed using co-occurrence networks and multidimensional scaling methods to identify the characteristics of each item, with data categorized in the SOAP format. Co-occurrence network analysis of NR1 in palliative care medicine revealed associations with the term “treatment” and future-oriented terms such as “future” and “progress” for the category A. Common terms for categories A and P included “palliative” and “hope,” while “pain,” “visit,” “care,” and “outpatient” were relevant to category P (Figure 2). The findings suggest that admission to the PCU is the most frequent decision-making scenario, indicating that patients maintain hope even as their condition progresses. In the multidimensional scaling analysis, the vertical and horizontal axes represent the degree and setting of treatment, respectively (Figure 3). The results indicate that the OCNSs facilitated decisionmaking support by alleviating patient concerns regarding medication, physical condition, and hospitalization (relevant to S and O), through providing simple explanations about treatment options and honoring the individual’s wishes.
Co-occurrence network analysis of NR1 for the Department of Breast Oncology revealed words such as “child,” “metastasis,” and “worry” for S. Regarding O and P, the terms “doctor,” “explanation,” “visit,” and “confirmation” emerged, while for P, words associated with communication such as “tell” and “consult” were noted. The word “genetics” appeared in the contexts of A and P, and “treatment,” “surgery,” and “examination” were present in each SOAP record (Figure 4). Words such as “child,” “transition,” and “concern” were encountered, suggesting that the OCNS was providing decision-making support regarding “genetic testing” and “treatment choice.” In multidimensional scaling, the vertical axis denotes the treatment target, while the horizontal axis reflects the state of mind (Figure 5). These results indicate that the OCNSs were sensitive to the patients’ and their families’ concerns and anxieties regarding breast cancer treatment options and the need for genetic testing.
At the beginning of the interview process for palliative care internal medicine, patients commonly sought information about their options and reported experiencing symptoms, as well as difficulty envisioning their future lives. However, after receiving decision-making support, a notable improvement was evident in the patients’ ability to make autonomous decisions and their acceptance of the illness and symptoms (Figure 6). In contrast, some individuals initially struggled to identify ways to achieve their goals. At the start of the process of interviewing with a breast oncology specialist, patients commonly sought information about their options, expressed goals they hoped to achieve, had trouble visualizing their future lifestyle, and reported symptoms. Following decision-making support, for each of these areas, improvement was observed in about 90% or more of cases (Figure 6).
In our analysis of palliative care and internal medicine SOAP records, we identified words associated with the future, such as “treatment,” “future,” “progress,” “alleviation,” and “hope,” in category A. In contrast, words like “pain,” “visit,” “care,” “outpatient,” “alleviation,” and “hope” were found in category P. This discrepancy may exist because “hospitalization in the PCU” often represents a pivotal decision-making point, in which patients find hope despite the progression of their illness. Recent advancements in model development, including OpenAI’s ChatGPT, have opened new avenues for the efficient curation of EHRs. Models that use ML extraction techniques for databases derived from EHRs do not predict future events or impute missing information. Instead, they offer a method for extracting clinically meaningful information—contextual details rather than just clinical keywords— from documents [17]. This technology has the potential to improve the validity of assessments by automatically and efficiently extracting information from EHRs indicating the need for medical intervention. This approach is particularly beneficial for patients whose cancer has progressed to the point where palliative care is more prevalent than active treatment, as the S and O (or SO) information in the EMR provides more contextual insight into their lives and emotions.
Regarding decision-making support, SO was used to identify patients’ concerns about their medications, physical condition, and hospitalization, and to present treatment options while offering support that respected the patient’s preferences. However, the narrative communication and mental health-related content exchanged between patients and nurses during the support process were not captured in the nursing records. Evaluations of EMRs in the mental health domain have highlighted benefits such as reduced adverse events and better quality of care [18]. Accessing EHRs and gaining a deeper understanding of mental health issues can facilitate the provision of appropriate support [19]. Additionally, the inclusion of a mental health care plan in EHR systems has been associated with a lower risk of attempted suicide [20]. However, challenges arise from the lack of mental health-related information in these systems, potentially disrupting workflows [21]. Another limitation is that EHRs do not capture information obtained through narrative communication, such as details about a patient’s life, social connections, and personal values [22], which can be burdensome to document and review [19]. Appropriate support is feasible when mental health information is available in the EHR; however, concerns persist regarding the labor-intensive process of converting and interpreting these data. Therefore, a prevailing strategy is to equip the EHR with artificial intelligence that can automatically analyze the mental health information contained within it, screen patients who need assistance, and alert healthcare professionals through the EHR. Depressive tendencies may arise in patients with cancer who require more palliative care. Early detection of depressive tendencies from the SO information recorded in the EMR is effective for early intervention by applying mental health knowledge to patients with cancer.
Even after receiving decision-making support, some individuals still face challenges in identifying pathways to achieve their goals, envisioning their future lives, or accepting their illness or symptoms. An EHR-embedded colorectal cancer (CRC) screening decision support program, termed “e-assist: Colon Health,” has been developed to facilitate informed decision-making in this context. This program is designed to effectively address common barriers and questions related to CRC screening, bridging the communication gap between healthcare providers and patients [23]. The integration of such a program into the EHRs of palliative care outpatient clinics is promising for establishing a systematic approach to advance care planning from the outset, such as at the time of cancer diagnosis. However, while bridging the gap may increase the likelihood of promoting cancer screening behavior, advance care planning requires addressing the emotional responses to the unexpected situations that arise with disease progression.
In an analysis of SOAP records from the breast oncology department, words such as “child,” “metastasis,” and “concern” may have been prominent due to the frequent inclusion of “genetic testing” and “treatment selection” in the decision-making scenarios. An EMR-based RWD collection framework has been designed to evaluate the efficacy and safety of anticancer drugs [24]. A retrospective observational analysis of EMR data in patients with small cell lung cancer showed that those who experienced myelosuppressive adverse events had a greater need for supportive care interventions and more frequent visits to inpatient and outpatient facilities [25]. Patient-generated health data, collected using smart devices or digital health technologies, are key to self-care and shared clinical decision-making. Studies have shown that free text and unstructured data can be processed and analyzed using NLP techniques to produce meaningful summaries and insights [26]. Thus, more efficient decisionmaking support can be achieved by leveraging information gathering and free text analysis methods focused on adverse event management. However, genetic tests performed in breast oncology departments may include information related to the risk of hereditary tumors, which requires counseling; hence, careful attention must be paid to information management. Notably, perceptions of family and individuals differ between Japan and the West; therefore, responses must be tailored to cultural backgrounds.
The observed improvement in patient reactions following the provision of decision-making support may be due to medical personnel’s recognition of the concerns and anxieties of patients and their families. This is supported by their provision of thorough explanations about breast cancer treatment options, including surgery, and the need for genetic testing. Previous research has shown that recording social determinants of health in the EHR and using NLP to extract this data from narrative clinical notes can be instrumental in creating screening tools, risk prediction models, and clinical decision support systems [27]. Therefore, carefully choosing the information to document in the EHR and utilizing NLP can represent an effective strategy for providing personalized care that addresses the needs of patients and their families. After receiving decision support, some individuals may still struggle to achieve their goals, manage symptoms, or accept their illness and symptoms. A system must be established that can identify such patients and connect them to advanced decision support resources. Incorporating patient-tailored interventions into the EHR to promote CRC screening can facilitate practice integration. However, the program’s effectiveness could be compromised without proper segmentation of the patient population [28]. Therefore, when implementing a decision support program within an EHR, it is essential to include a screening function that categorizes the patient population based on specific information.
The study’s limitations are numerous decision-making scenarios related to genetic testing within the breast oncology department and admission to the palliative care ward. Consequently, the nursing records may have contained more contextual expressions to describe the phenomenon rather than concise information. This might have obscured the results of the analysis and hindered the identification of trends.
In summary, decision-making within the palliative care department frequently revolved around admission to the PCU. This suggests that patients continue to find hope as their disease progresses. Regarding decision-making support, the SO framework was utilized to collect patients’ concerns regarding medication, physical condition, and hospitalization, and to present treatment options that respected patient preferences. Decision-making in the breast oncology department often focused on “genetic testing” and “treatment options.” In the context of decision support, SO clearly played a key role in providing information and facilitating communication related to genetics. However, the development of a decision-making program that leverages EMRs requires further research. This research should aim to categorize the patient population based on EMR data and to identify markers indicating patients who will require care.
Acknowledgments
I am grateful to Satomi H., Hitomi M., and Naoko O. for their collaboration in the early stages of this work. This research was supported by JSPS KAKENHI (Grant No. 23K24651).
References
1. Ministry of Health, Labor and Welfare. Trends in the spread of electronic medical record systems, etc. [Internet]. Tokyo, Japan: Ministry of Health, Labor and Welfare;2023. [cited at 2024 May 9] Available from: https://www.mhlw.go.jp/content/10800000/000938782.pdf.
2. Zhao Y, Tsubota T. The current status of secondary use of claims, electronic medical records, and electronic health records in epidemiology in Japan: narrative literature review. JMIR Med Inform. 2023; 11:e39876.
https://doi.org/10.2196/39876.

3. Hernandez MN, Voti L, Feldman JD, Tannenbaum SL, Scharber W, Mackinnon JA, et al. Cancer registry enrichment via linkage with hospital-based electronic medical records: a pilot investigation. J Registry Manag. 2013; 40(1):40–7.
4. Weiskopf NG, Weng C. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc. 2013; 20(1):144–51.
https://doi.org/10.1136/amiajnl-2011-000681.

5. Lewis AE, Weiskopf N, Abrams ZB, Foraker R, Lai AM, Payne PR, et al. Electronic health record data quality assessment and tools: a systematic review. J Am Med Inform Assoc. 2023; 30(10):1730–40.
https://doi.org/10.1093/jamia/ocad120.

6. Kruse CS, Stein A, Thomas H, Kaur H. The use of electronic health records to support population health: a systematic review of the literature. J Med Syst. 2018; 42(11):214.
https://doi.org/10.1007/s10916-018-1075-6.

7. Townsend JS, Jones MC, Jones MN, Waits AW, Konrad K, McCoy NM. A case study of early-onset colorectal cancer: using electronic health records to support public health surveillance on an emerging cancer control topic. J Registry Manag. 2021; 48(1):4–11.
8. Gensheimer MF, Narasimhan B, Henry AS, Wood DJ, Rubin DL. Accuracy of electronic medical record follow-up data for estimating the survival time of patients with cancer. JCO Clin Cancer Inform. 2022; 6:e2200019.
https://doi.org/10.1200/CCI.22.00019.

9. Sollie A, Sijmons RH, Helsper C, Numans ME. Reusability of coded data in the primary care electronic medical record: a dynamic cohort study concerning cancer diagnoses. Int J Med Inform. 2017; 99:45–52.
https://doi.org/10.1016/j.ijmedinf.2016.08.004.

10. Conderino S, Bendik S, Richards TB, Pulgarin C, Chan PY, Townsend J, et al. The use of electronic health records to inform cancer surveillance efforts: a scoping review and test of indicators for public health surveillance of cancer prevention and control. BMC Med Inform Decis Mak. 2022; 22(1):91.
https://doi.org/10.1186/s12911-022-01831-8.

11. Benedum CM, Sondhi A, Fidyk E, Cohen AB, Nemeth S, Adamson B, et al. Replication of real-world evidence in oncology using electronic health record data extracted by machine learning. Cancers (Basel). 2023; 15:1853.
https://doi.org/10.3390/cancers15061853.

12. Knevel R, Liao KP. From real-world electronic health record data to real-world results using artificial intelligence. Ann Rheum Dis. 2023; 82(3):306–11.
https://doi.org/10.1136/ard-2022-222626.

13. Khosravi B, Rouzrokh P, Erickson BJ. Getting more out of large databases and EHRs with natural language processing and artificial intelligence: the future is here. J Bone Joint Surg Am. 2022; 104(Suppl 3):51–5.
https://doi.org/10.2106/JBJS.22.00567.

14. Sim JA, Huang X, Horan MR, Stewart CM, Robison LL, Hudson MM, et al. Natural language processing with machine learning methods to analyze unstructured patient-reported outcomes derived from electronic health records: a systematic review. Artif Intell Med. 2023; 146:102701.
https://doi.org/10.1016/j.artmed.2023.102701.

15. Hossain E, Rana R, Higgins N, Soar J, Barua PD, Pisani AR, et al. Natural language processing in electronic health records in relation to healthcare decision-making: a systematic review. Comput Biol Med. 2023; 155:106649.
https://doi.org/10.1016/j.compbiomed.2023.106649.

16. Sim JA, Huang X, Horan MR, Baker JN, Huang IC. Using natural language processing to analyze unstructured patient-reported outcomes data derived from electronic health records for cancer populations: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2024; 24(4):467–75.
https://doi.org/10.1080/14737167.2024.2322664.

17. Adamson B, Waskom M, Blarre A, Kelly J, Krismer K, Nemeth S, et al. Approach to machine learning for extraction of real-world data variables from electronic health records. Front Pharmacol. 2023; 14:1180962.
https://doi.org/10.3389/fphar.2023.1180962.

18. Zurynski Y, Ellis LA, Tong HL, Laranjo L, Clay-Williams R, Testa L, et al. Implementation of electronic medical records in mental health settings: scoping review. JMIR Ment Health. 2021; 8(9):e30564.
https://doi.org/10.2196/30564.

19. Schwarz J, Barkas A, Blease C, Collins L, Hagglund M, Markham S, et al. Sharing clinical notes and electronic health records with people affected by mental health conditions: scoping review. JMIR Ment Health. 2021; 8(12):e34170.
https://doi.org/10.2196/34170.

20. Dutta R, Gkotsis G, Velupillai SU, Downs J, Roberts A, Stewart R, et al. Identifying features of risk periods for suicide attempts using document frequency and language use in electronic health records. Front Psychiatry. 2023; 14:1217649.
https://doi.org/10.3389/fpsyt.2023.1217649.

21. Kariotis TC, Prictor M, Chang S, Gray K. Impact of electronic health records on information practices in mental health contexts: scoping review. J Med Internet Res. 2022; 24(5):e30405.
https://doi.org/10.2196/30405.

22. Weir C. Through the narrative looking glass: commentary on “impact of electronic health records on information practices in mental health contexts: scoping review”. J Med Internet Res. 2022; 24(5):e38513.
https://doi.org/10.2196/38513.

23. Lafata JE, Shin Y, Flocke SA, Hawley ST, Jones RM, Resnicow K, et al. Randomised trial to evaluate the effectiveness and impact of offering postvisit decision support and assistance in obtaining physician-recommended colorectal cancer screening: the e-assist: colon health study-a protocol study. BMJ Open. 2019; 9(1):e023986.
https://doi.org/10.1136/bmjopen-2018-023986.

24. Han HS, Lee KE, Suh YJ, Jee HJ, Kim BJ, Kim HS, et al. Data collection framework for electronic medical record-based real-world data to evaluate the effectiveness and safety of cancer drugs: a nationwide real-world study of the Korean cancer study group. Ther Adv Med Oncol. 2022; 14:17588359221132628.
https://doi.org/10.1177/17588359221132628.

25. Epstein RS, Weerasinghe RK, Parrish AS, Krenitsky J, Sanborn RE, Salimi T. Real-world burden of chemotherapy-induced myelosuppression in patients with small cell lung cancer: a retrospective analysis of electronic medical data from community cancer care providers. J Med Econ. 2022; 25(1):108–18.
https://doi.org/10.1080/13696998.2021.2020570.

26. Sezgin E, Hussain SA, Rust S, Huang Y. Extracting medical information from free-text and unstructured patient-generated health data using natural language processing methods: feasibility study with real-world data. JMIR Form Res. 2023; 7:e43014.
https://doi.org/10.2196/43014.

27. Patra BG, Sharma MM, Vekaria V, Adekkanattu P, Patterson OV, Glicksberg B, et al. Extracting social determinants of health from electronic health records using natural language processing: a systematic review. J Am Med Inform Assoc. 2021; 28(12):2716–27.
https://doi.org/10.1093/jamia/ocab170.

28. Elston Lafata J, Shires DA, Shin Y, Flocke S, Resnicow K, Johnson M, et al. Opportunities and challenges when using the electronic health record for practice-integrated patient-facing interventions: the e-assist colon health randomized trial. Med Decis Making. 2022; 42(8):985–98.
https://doi.org/10.1177/0272989X221104094.

Figure 1
Frequencies of decisions on various matters: (A) internal medicine palliative care department and (B) breast oncology department. PCU: palliative care unit, DNAR: do not attempt resuscitation, CPR: cardiopulmonary resuscitation, BSC: best supportive care (provision of symptomatic relief alone without active cancer treatment). “Second opinion” refers to the request of a doctor’s opinion at another medical institution in addition to that of the currently treating physician.
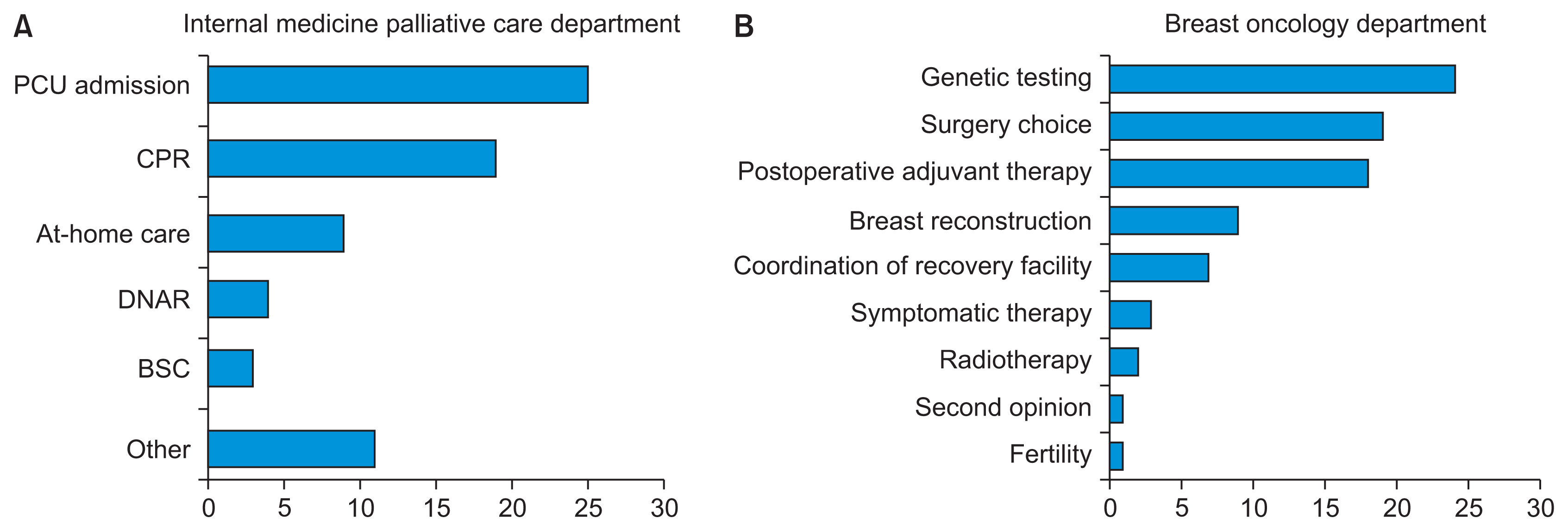
Figure 2
Nursing records on decision support within electronic medical records (internal medicine palliative care department): a co-occurrence network analysis. “Degree” represents grouping of similarities.
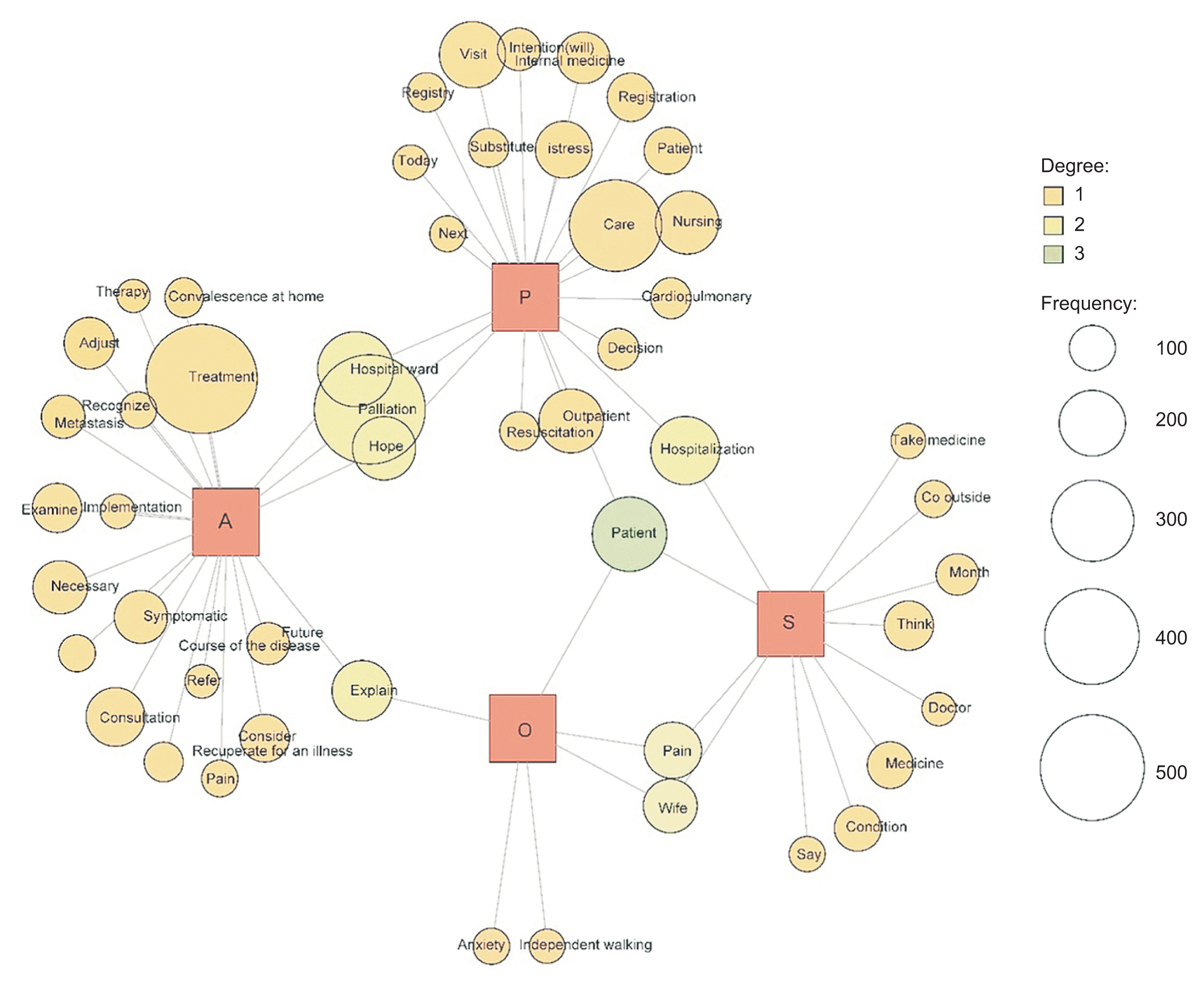
Figure 3
Nursing records on decision support within electronic medical records (internal medicine palliative care department): a multidimensional scaling analysis.
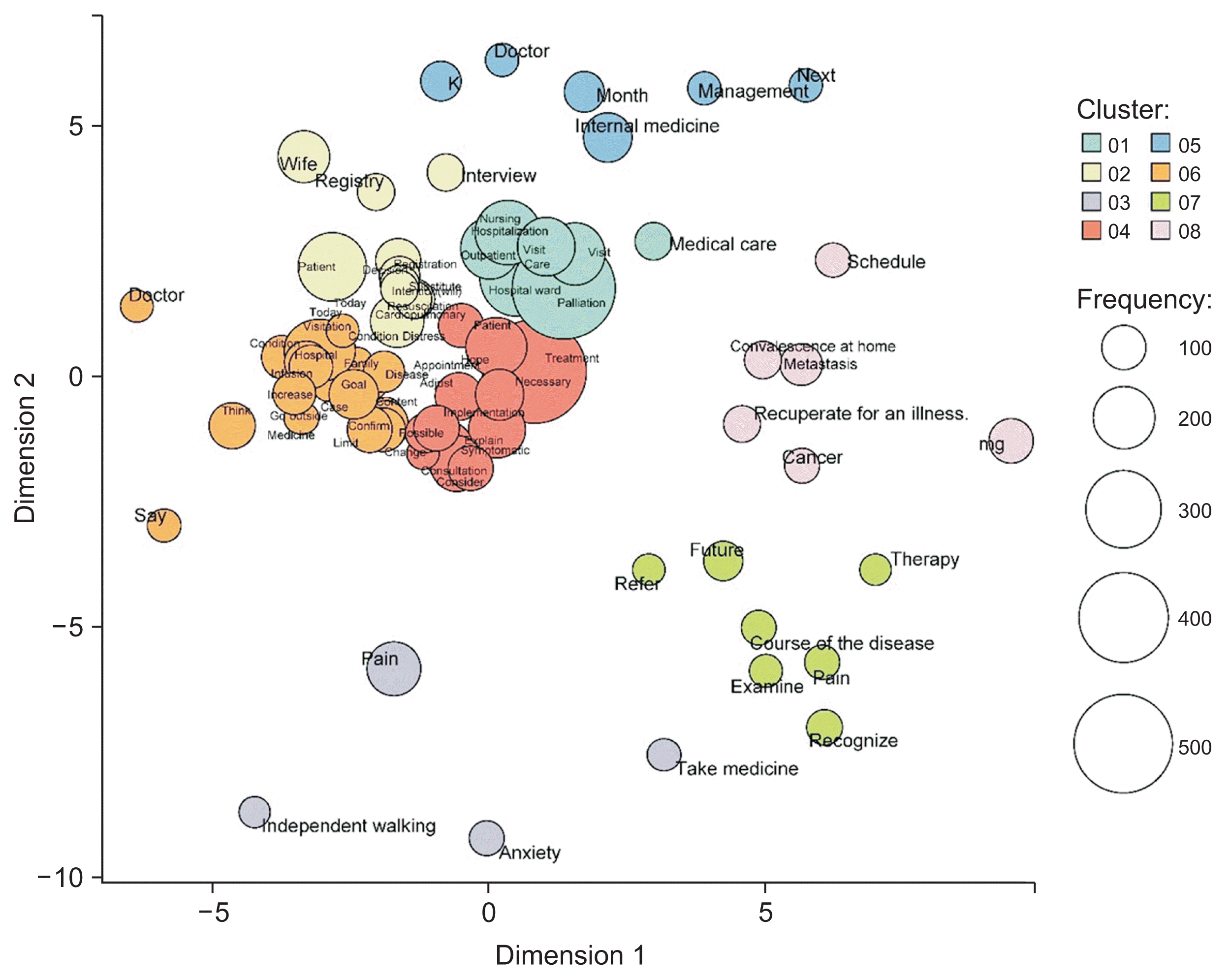
Figure 4
Nursing records on decision support within electronic medical records (breast oncology department): a co-occurrence network analysis. “Degree” represents grouping of similarities.
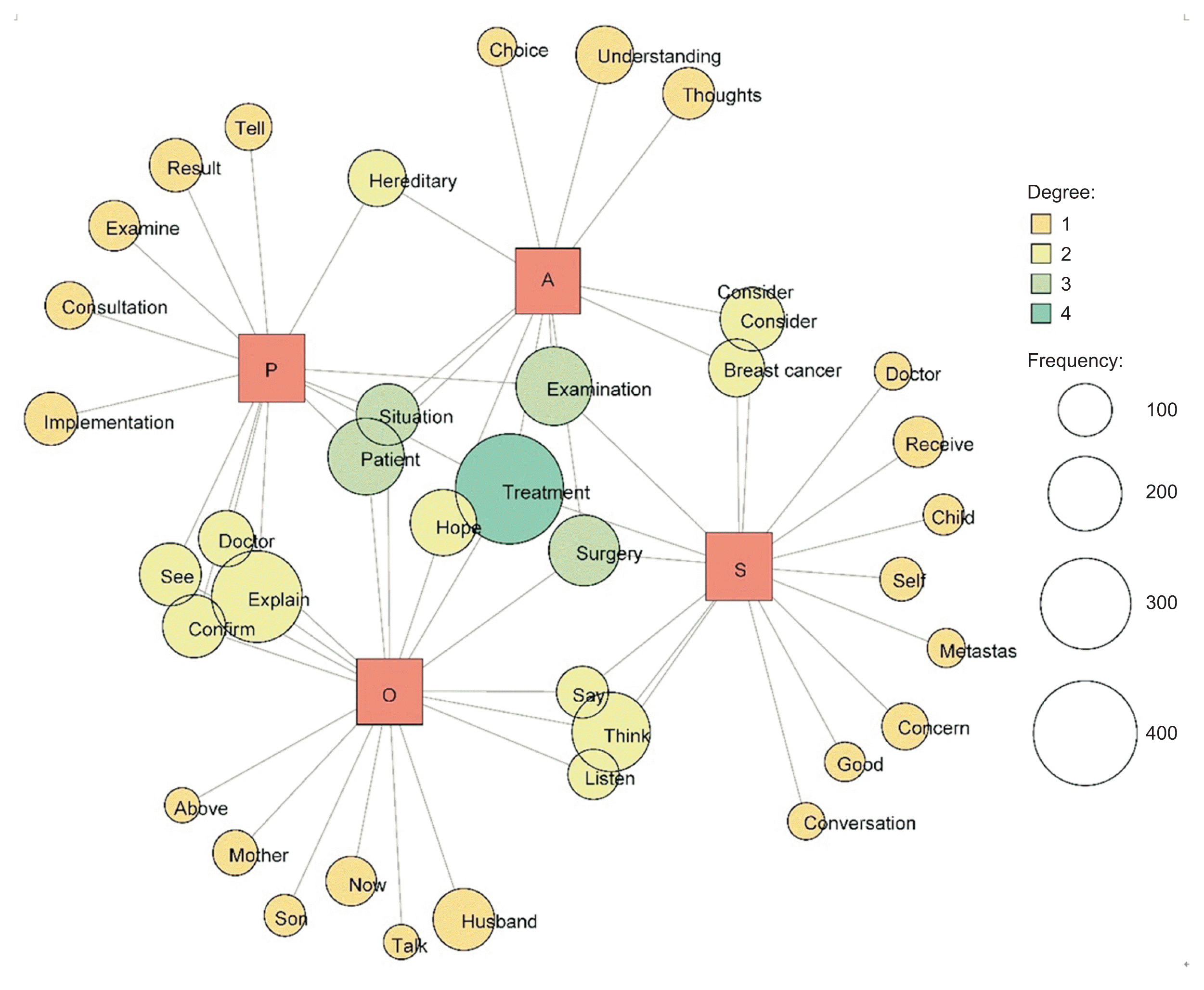
Figure 5
Nursing records on decision support within electronic medical records (breast oncology department): a multidimensional scaling analysis.
Figure 6
Changes in patients and families after decision support: (A) internal medicine palliative care department and (B) breast oncology department.
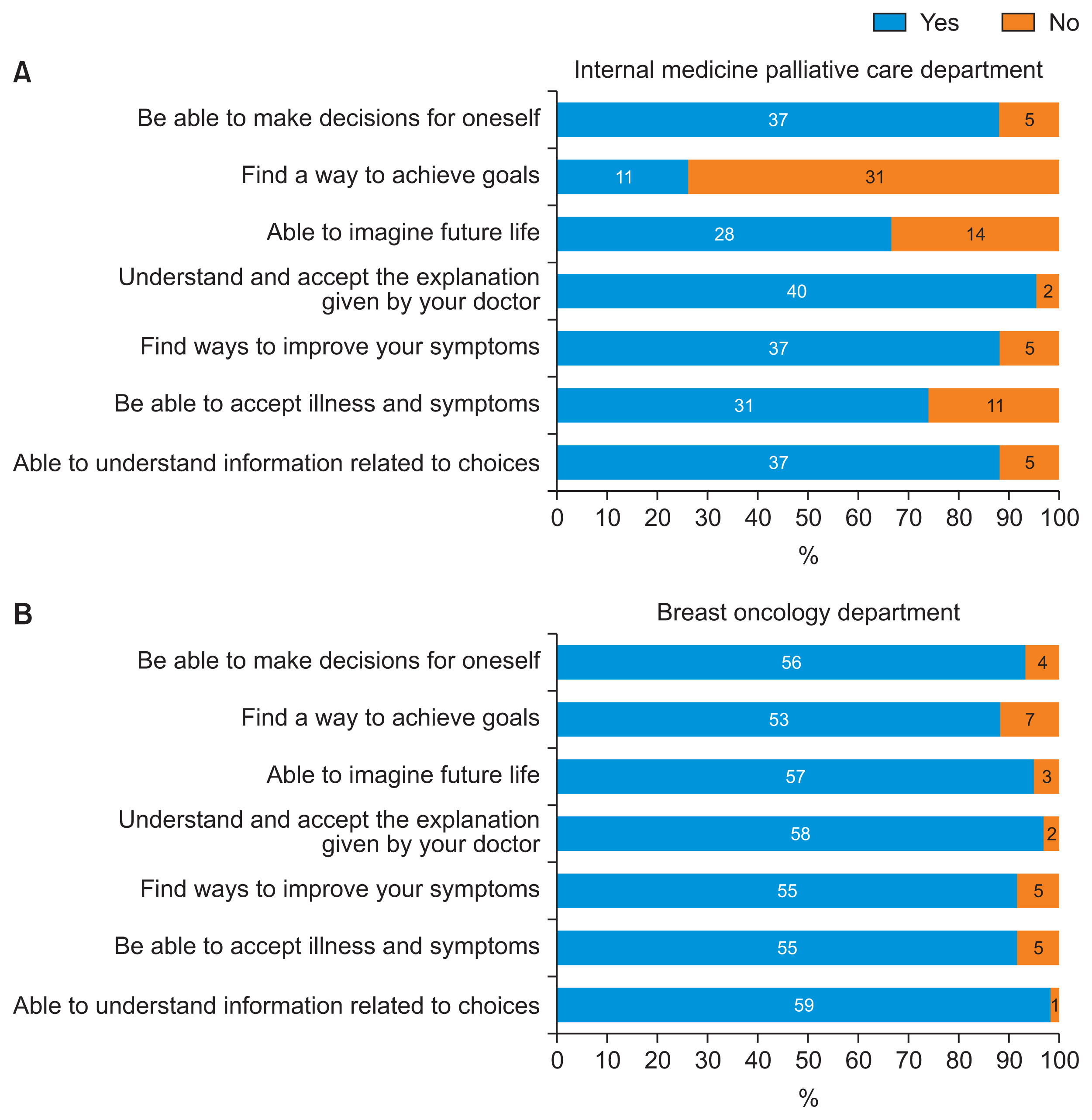
Table 1
Participant characteristics
| Internal medicine palliative care department (n = 42) | Breast oncology department (n = 60) | |
|---|---|---|
| Sex | ||
| Male | 24 (57) | 0 (0) |
| Female | 18 (43) | 60 (100) |
|
|
||
| Age (yr) | 70.5 ± 12.1 | 55.8 ± 12.2 |
|
|
||
| Metastasis | ||
| Yes | 18 (43) | 7 (12) |
|
|
||
| Recurrence | ||
| Yes | 5 (12) | 1 (2) |
|
|
||
| Site | ||
| Breast | 4 (10) | 60 (100) |
| Pharynx | 1 (2) | 0 (0) |
| Esophagus | 1 (2) | 0 (0) |
| Digestive system | 14 (33) | 0 (0) |
| Liver/gallbladder/pancreas | 6 (14) | 0 (0) |
| Lung | 3 (7) | 0 (0) |
| Urinary system | 4 (10) | 0 (0) |
| Lymph nodes | 2 (5) | 0 (0) |
| Ovaries | 1 (2) | 0 (0) |
| Thyroid | 1 (2) | 0 (0) |
| Duodenum | 1 (2) | 0 (0) |
| Appendix | 1 (2) | 0 (0) |
| Peritoneum | 1 (2) | 0 (0) |
| Anus | 1 (2) | 0 (0) |
| Other | 1 (2) | 0 (0) |
|
|
||
| Companion | ||
| Yes | 38 (90) | 49 (82) |
|
|
||
| Effect on decision-makinga) | ||
| Family | 6 (14) | 11 (18) |
| Medical professionals | 5 (12) | 4 (7) |
| Patient themselves | 28 (67) | 44 (73) |
|
|
||
| Involvement in decision-makinga) | ||
| Family | 33 (79) | 54 (90) |
| Medical professionals | 38 (90) | 57 (95) |
| Patient themselves | 38 (90) | 50 (83) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download