Glossary
Abbreviations
EBV +
DLBCL
R/R
PD-L1
OS
IHC
ISH
GCB
EBERs
FDG- PET/CT
CT
qPCR
PFS
CR
PR
R-CHOP
R-ICE
ICE
PD
ORR
CRR
BV
ECOG PS
LDH
IPI
irADRs
Journal List > Blood Res > v.59 > 1516088900
EBV +
DLBCL
R/R
PD-L1
OS
IHC
ISH
GCB
EBERs
FDG- PET/CT
CT
qPCR
PFS
CR
PR
R-CHOP
R-ICE
ICE
PD
ORR
CRR
BV
ECOG PS
LDH
IPI
irADRs
EBV +
DLBCL
R/R
PD-L1
OS
IHC
ISH
GCB
EBERs
FDG- PET/CT
CT
qPCR
PFS
CR
PR
R-CHOP
R-ICE
ICE
PD
ORR
CRR
BV
ECOG PS
LDH
IPI
irADRs
Authors’ contributions
GLC, JNC, and FFL: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, and Writing – review & editing. YLL: Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, and Writing – review & editing. YHW and CSF: Data curation, Formal analysis, Methodology, Validation, Visualization, and Writing – original draft. BHY: Data curation, Methodology, Supervision, Visualization, and Writing – original draft. QLZ and ZGX: Data curation, Methodology, Supervision, Validation, and Writing – original draft. All authors read and approved the final manuscript.
Ethical approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center (ZRB1612167-18). The requirement for informed consent was waived for this retrospective study.
Author details
1 Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, P.R. China. 2 Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, P.R. China. 3 Department of Medical Oncology, Fudan University Shanghai Cancer Center Xiamen Hospital, Xiamen 361026, P.R. China. 4 Department of Pathology, Fujian Province, Zhangzhou Affiliated Hospital of Fujian Medical University, Zhangzhou 363000, P.R. China. 5 Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai 200032, P.R. China. 6 Present Address: Department of Nursing, Fudan University Shanghai Cancer Center, Shanghai 200032, P.R. China.
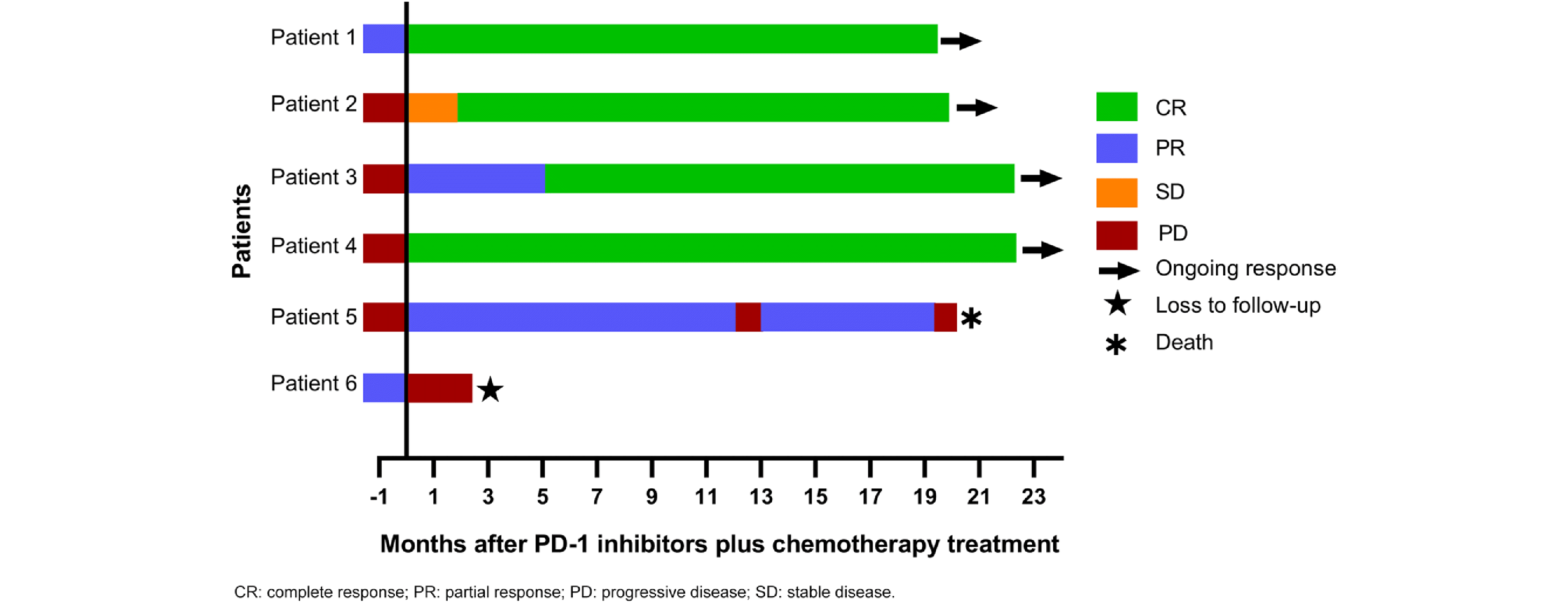
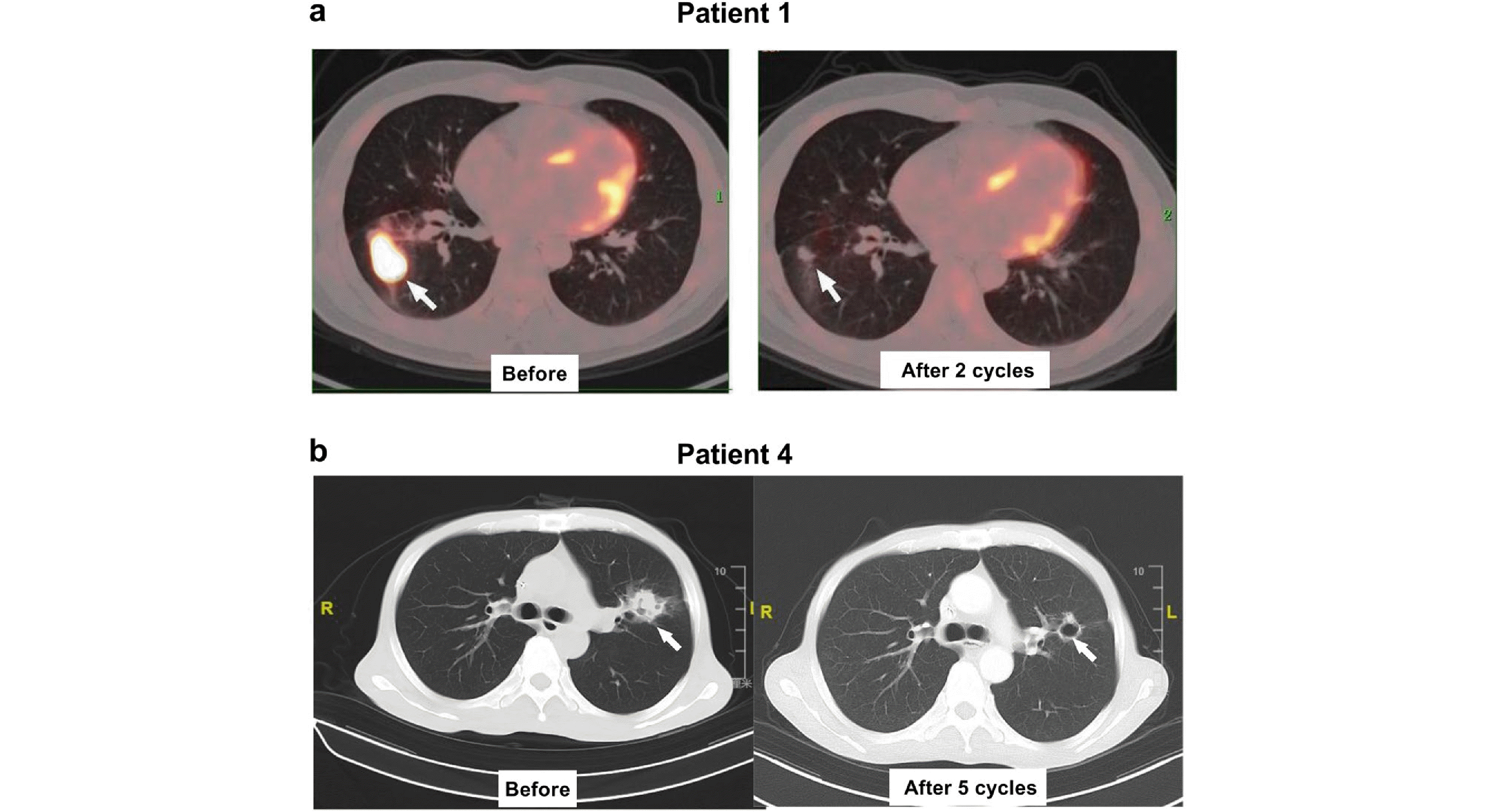
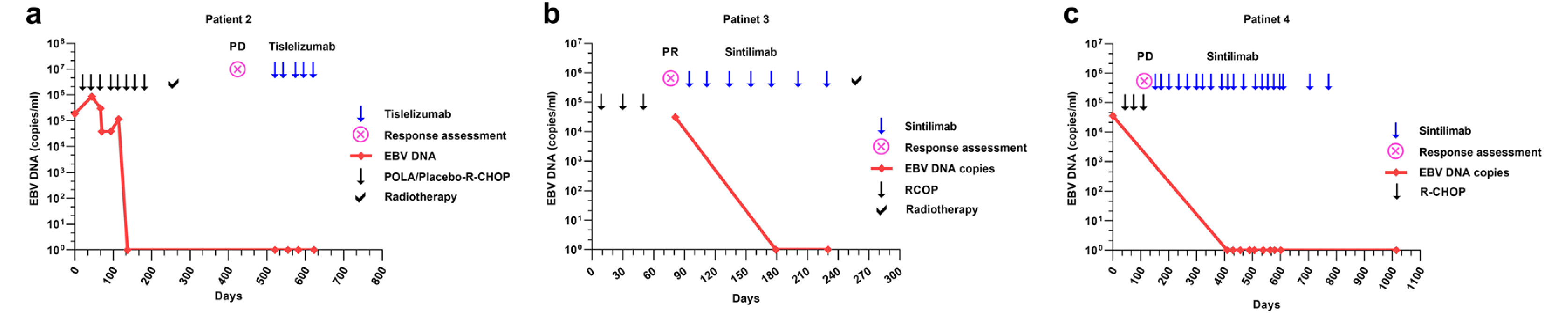
R-CHOP rituximab, cyclophosphamide, vindesine, doxorubicin, prednisone, R-GP rituximab, gemcitabine, cisplatinum, R-ICE rituximab, ifosfamide, carboplatin, and etoposide, G gemcitabine, P cisplatinum, CR complete response, PR partial response, PD progressive disease, OS Overall Survival, PFS progression-free survival, irADRs immune-related adverse drug reactions