This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
This study aimed to develop a flexible eye shield phantom to acquire artifact-free computed tomography (CT) images for electron beam radiotherapy.
Methods
A flexible eye shield phantom for a newly designed eye shield was fabricated. Because of metal artifacts caused by an eye shield composed of high-density materials such as tungsten or lead, CT image acquisition is not appropriate for treatment planning because of inaccurate dose calculation and organ-at-risk delineation. To acquire artifact-free CT images, a mold of the same size as the outer dimension of the metallic eye shield was manufactured using 3D printing. The flexible eye shield phantom was imaged using a Philips Brilliance CT Big Bore under the same condition as the measurement. The phantom image with an average of 200 Hounsfield unit (HU) was imported into the treatment planning systems (TPS) and assigned a value of 26,750 HU to consider the material density of tungsten. The dosimetric comparison using a 6-MeV electron beam was performed. Measurement was performed using a metal oxide semiconductor field effect transistor detector for point doses at 3 and 10 mm.
Results
The artifact-free CT images using a flexible eye shield phantom without air bubbles were transferred into the TPS. The dose at 10 mm calculated using the TPS agreed with the ion-chamber measurements within 2 cGy. Conversely, a larger dose discrepancy between the measured and calculated doses was found at 3 mm depth.
Conclusion
The flexible eye shield phantom was successfully fabricated to apply electron treatment planning by acquiring artifact-free CT images. The dose calculated using the artifact-free image was comparable to the measured dose at lens depth when applying an eye shield.
Keywords: Eye shielding, Flexible eye shield phantom, Artifact-free CT image
Introduction
Eyelid lesions necessitate the use of metallic eye shields in radiation therapy to minimize complications such as cataract, keratopathy, retinopathy, and optic neuropathy [
1-
5]. Proposed for electron beam or kilo-voltage photon beam treatments, metallic eye shields made of high Z materials such as lead or tungsten should meet three essential requirements when positioned between the eyeball and the eyelid: (1) harmless to normal orbital tissues, (2) sufficient protection for the cornea and lens, and (3) no attenuation of the local therapeutic effect by partially shielding the planning target volume. Accurate estimation of the dose delivered to the surrounding area is crucial when using metallic eye shields [
6,
7]. However, the presence of a metallic eye shield in computed tomography (CT) images poses challenges in treatment planning systems (TPS) because of difficulties in acquiring precise CT numbers and the delineation of surrounding tissues caused by severe metal artifacts. To address this, a nonmetallic dummy shield was fabricated as a substitute for metallic eye shields. Studies have shown the successful implementation of machined acrylic dummy shields for accurate dose calculation using a commercial TPS with CT-based planning and Monte Carlo (MC) simulation [
8]. In addition, the fabrication of the dummy eye shields using 3D scanning and printing techniques has been demonstrated, showing effective adoption for treating ocular tumors with electron radiotherapy [
9,
10]. However, dummy eye shields made of 3D printing materials such as polylactic acid or acrylonitrile butadiene styrene exhibit shore hardness levels of ≥70D, potentially causing eye damage. Consequently, additional packaging is required to avoid direct eye contact [
10].
In this study, a flexible eye shield phantom was fabricated using a 3D-printed mold and casting method. The dose distribution calculated using the flexible dummy shield with an assigned CT value was compared with the dose measured with a metal shield using a metal oxide semiconductor field effect transistor (MOSFET) detector.
Materials and Methods
1. New design of the tungsten eye shield
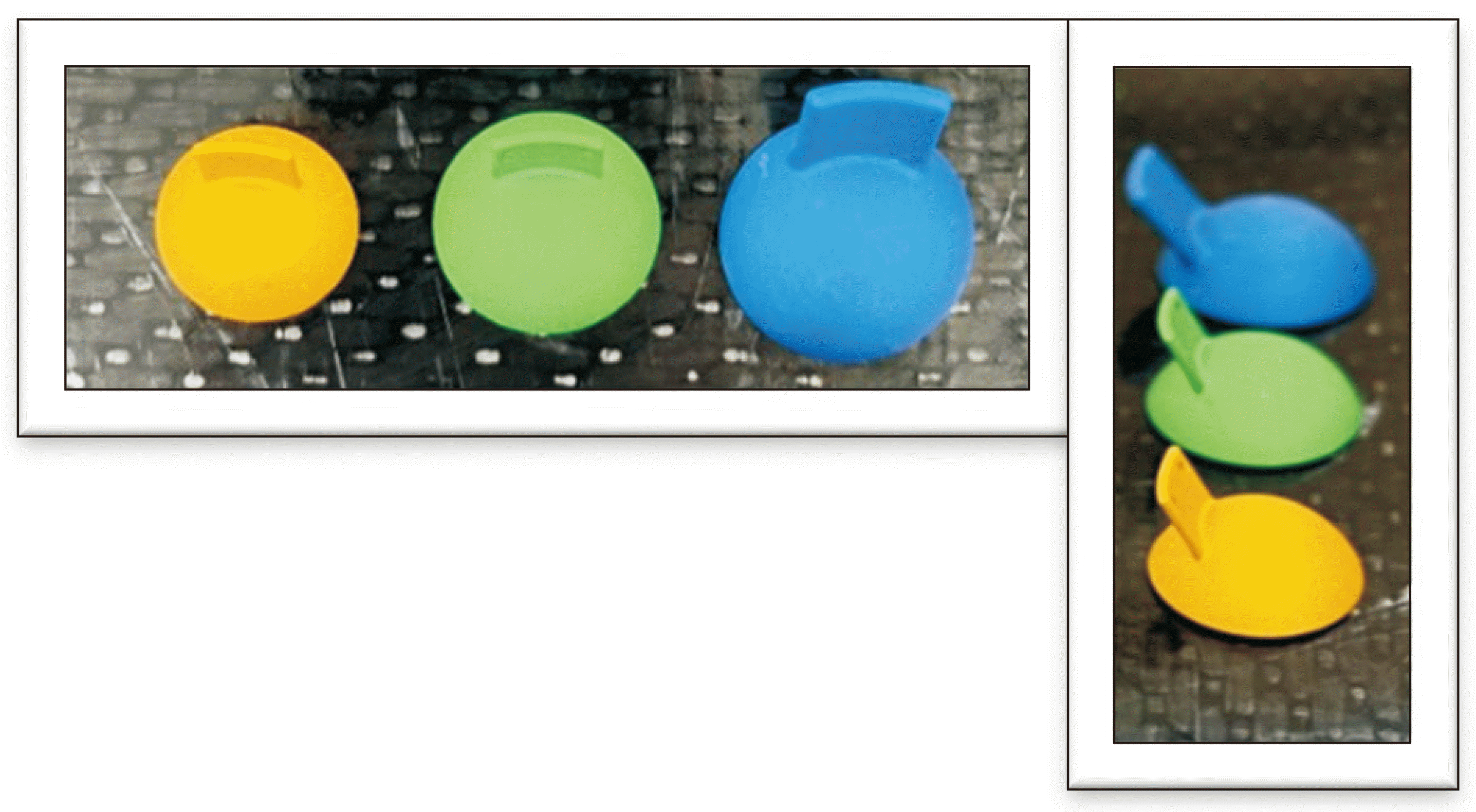
Fig. 1 shows the newly designed eye shields with an aluminum cap, which were labeled small, medium, and large, to minimize backscatter dose, and their physical properties are summarized in
Table 1. According to a previous study, the optimum thickness of the eye shields made of tungsten and aluminum for electron radiotherapy is 2 or 3 mm of tungsten with an aluminum cap of 0.5 or 1 mm. The newly designed eye shields were composed of 3 mm of tungsten and 1 mm of aluminum. The aluminum cap can be fastened to the tungsten body as a screw connection, and if the aluminum cap is not used, the detachable handle can be combined with the tungsten body. The eye shields can be easily sterilized by gas, and the anodized aluminum cap is mechanically and chemically durable. Compared with existing products that have a steel knob at the center, when an aluminum cap is used, the position of the handle is shifted to one side as shown in
Fig. 1.
2. Fabrication of the flexible eye shield phantom
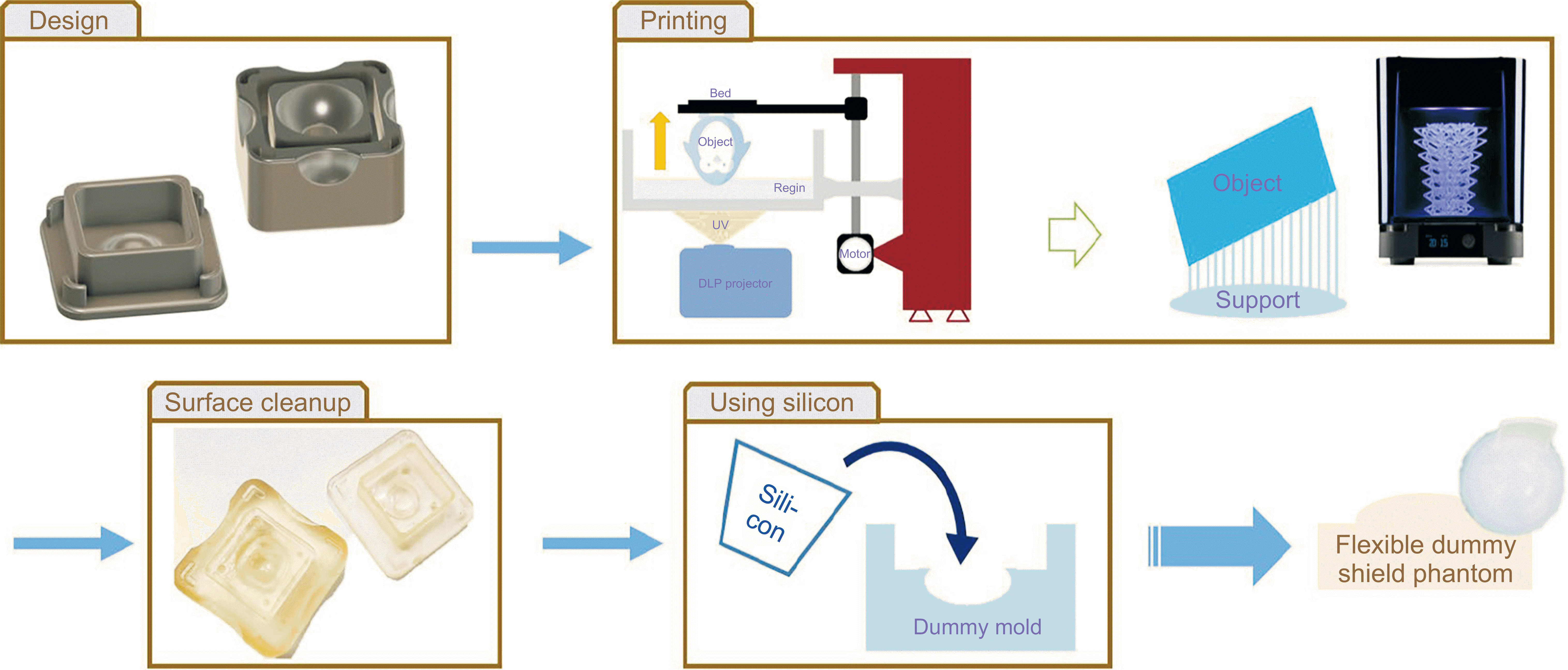
The comprehensive fabrication process for the flexible eye shield phantom is illustrated in
Fig. 2. The flexible eye shield phantoms for the newly designed eye shields in three different sizes were manufactured. Instead of employing direct 3D printing, silicone was cast into 3D-printed molds to create the flexible eye shield phantom. The mold, matching the outer dimensions of the metallic eye shield, was produced using digital light 3D printing. After printing the mold, supports were removed. Certified harmless silicone, approved for safety and skin sensitization testing, was poured into the 3D-printed mold. Subsequently, the mixture was cured at room temperature for approximately 4 hours within an in-house vacuum chamber to eliminate air bubbles. Finally, the mold was meticulously removed by cutting from the cured flexible eye shield phantom.
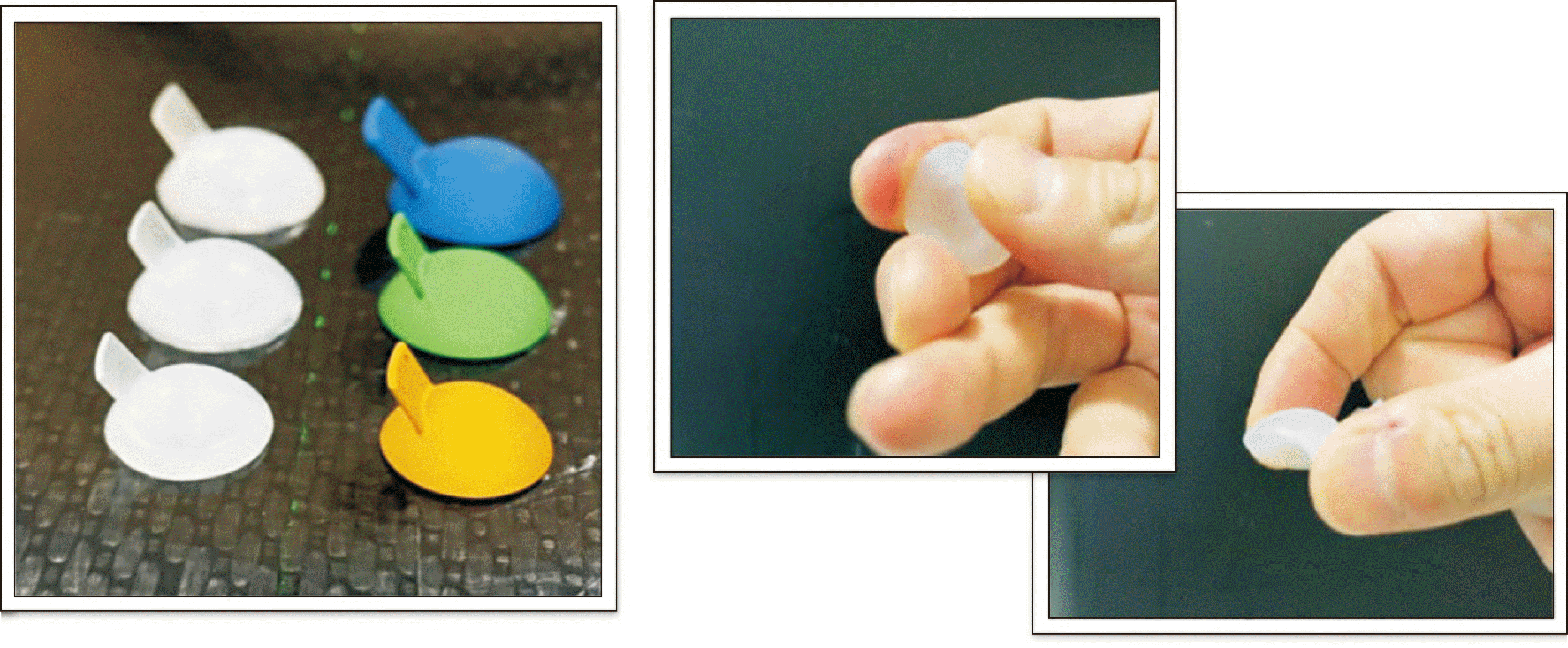
Fig. 3 displays the resulting flexible eye shield phantom.
3. CT scan and dosimetric evaluation
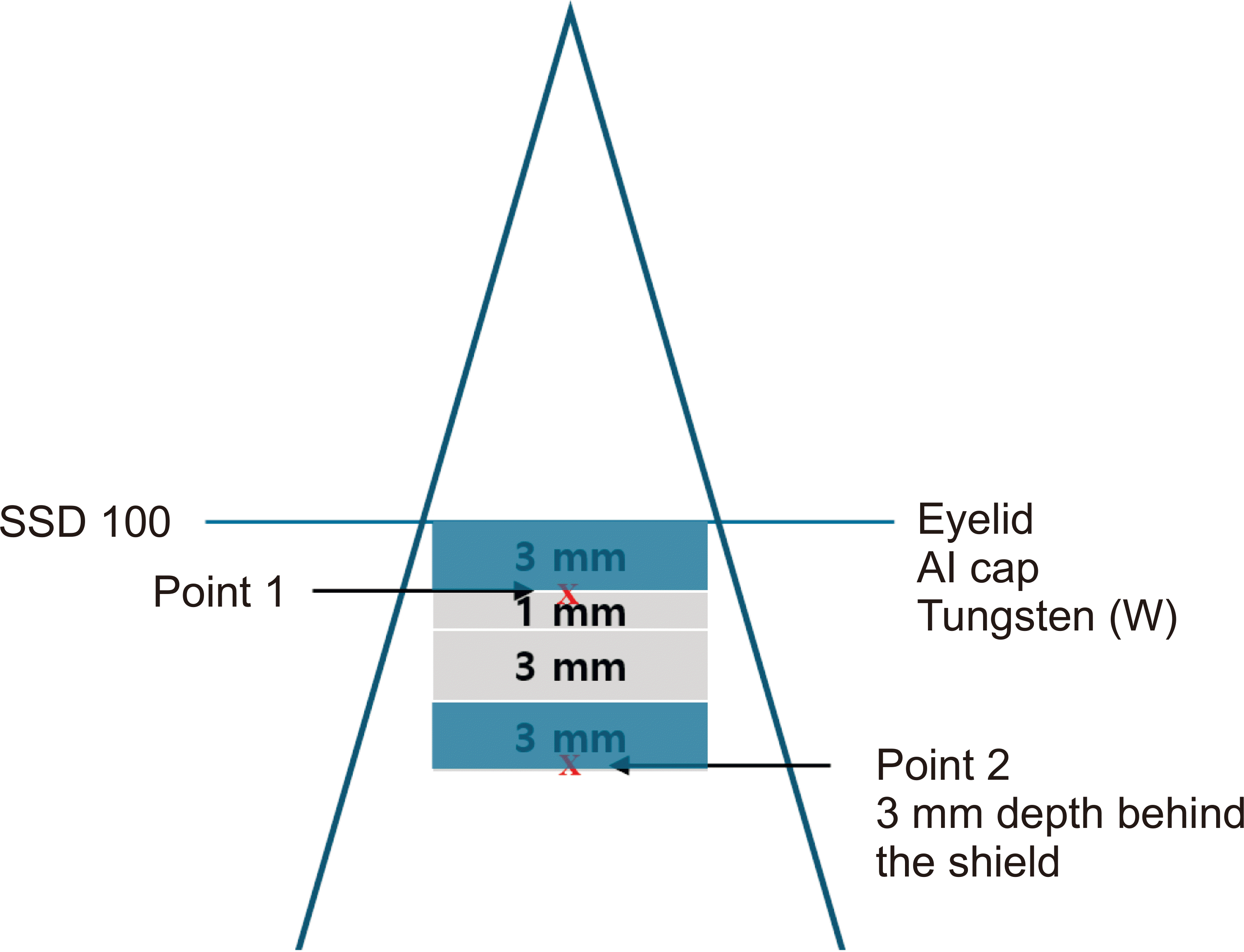
The fabricated flexible eye shield phantom was scanned with a Brilliance Big Bore CT simulator (Philips Inc.) for dose calculation using TPS with CT-based treatment planning. For eyelid simulation, a 3-mm bolus was used to cover the eye shield above the 5-cm solid water phantom. CT was performed with a slice thickness of 1 mm, and the acquired CT images were transferred to the TPS for dose calculation. The eye shield was delineated using a drawing tool with a high-resolution segment. In the planning, tungsten and aluminum were used for density override, and each was assigned a Hounsfield unit (HU) of 26,750 and 2,599, along with material densities of 17 and 2.7 g/cm³, respectively. The dose calculation with the flexible eye shield phantom was performed using the electron MC (eMC) algorithm with a grid size of 0.25 cm and statistical uncertainty of 2 using Eclipse TPS (version 16.1; Varian Medical System). A 6-MeV electron beam was used with a fixed source-to-surface distance of 100 cm and 10 cones for 100 monitor units (MUs). To evaluate the calculated dose, two points were measured using MOSFET as shown in
Fig. 3. To simulate the thickness of the eyelid, the first point (point 1) was placed on top of the aluminum cap with 3 mm of the bolus above the MOSFET detector. Point 2, which simulated the depth to the anterior aspect of the lens, was positioned at a depth of 3 mm behind the eye shield.
Results
Fig. 4 shows flexible eye shield phantom which has the same appearance as the newly designed eye shield consisting of small, medium, and large size. The silicone used in the flexible eye shield phantom which is harmless, does not generate metal artifacts in CT images, so it is not only advantageous for delineating the surrounding organ-at-risk (OAR) and target volume, but also resolve error in CT-based dose calculations caused by metal artifacts.
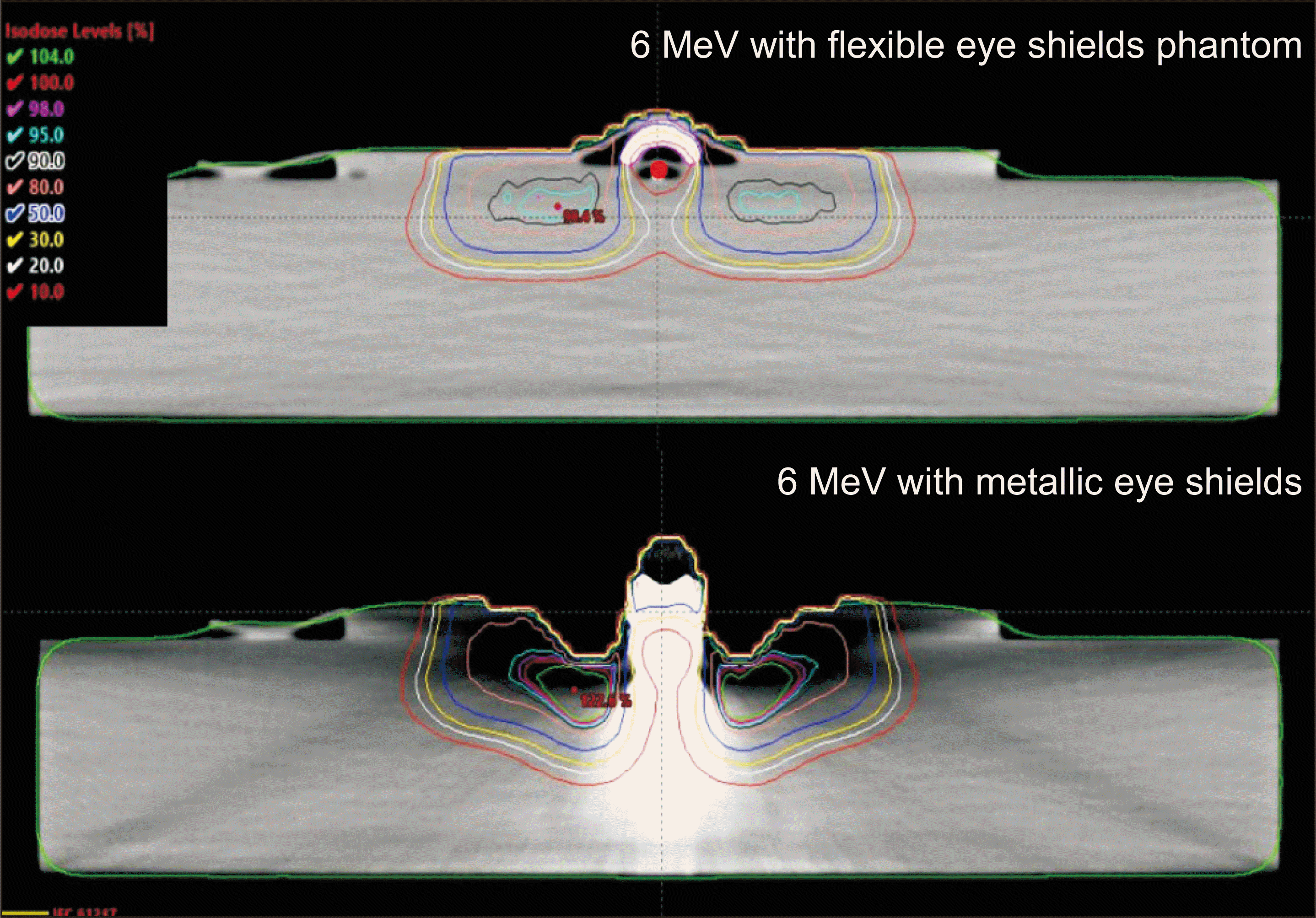
Fig. 5 illustrates the differences in dose distribution between when performing dose calculations based on CT images acquired using flexible eye shield phantom and CT images using the eye shield made by high density material. The HU value corresponding to tungsten was overridden in the flexible eye shield phantom, and a 6-MeV beam with 100 MU was irradiated.
Table 2 compares dose calculated by the eMC algorithm with flexible eye shield phantom to dose measured by MOSFET. A 3-mm bolus was used to simulate the eyelid depth, resulting in mean differences between measured and calculated doses at the eyelid of 36.2%, 34.2%, and 34.1% for small, medium, and large sizes, respectively. In contrast, at the position for measuring transmitted dose beyond the eye shield, the mean differences were 1.3, 0.9, and 0.8 cGy for small, medium, and large sizes, respectively.
Discussion
Compared with the traditional rigid acrylic material dummy, the flexible eye shield phantom may provide greater comfort to patients and to potentially reduce the risk of eye damage. Moreover, the relatively low cost of manufacturing a flexible eye shield phantom using silicone compared to processing acrylic makes it more suitable for single-patient use to prevent infections. The dose distribution similar to previous studies was acquired by using CT imaging without metal artifact. On the contrary, dose evaluation was made difficult by metal artifact when performing dose calculation using CT image with the eye shield. The MOSFET measurements showed relatively good agreement at a depth of 10 mm simulating the lens depth. These results were consistent with previous studies comparing calculated dose using acrylic dummy shield [
7-
9]. In addition, Kang et al. [
7,
8] showed a difference of approximately −20% in the dose at the eyelid compared with the MC results in a commercial TPS using a pencil beam algorithm, which is similar to the underestimated dose at the eyelid as shown in this study. These results indicated that dummy shields can be applicable for estimating the dose below the shield in TPS; however, they may be inappropriate for evaluating eyelid doses in clinical practice. These results can be inferred from the finding that the eMC algorithm, as shown in previous studies, cannot properly consider backscatter from higher density materials [
11]. In this study, the HU was assigned to extremely high-density materials such as tungsten, which could have emphasized the discrepancies. Therefore, for accurate assessment of doses around high Z materials, using a fully MC calculation is recommended rather than using an algorithm that provides mass densities of 5 preset materials. In the future, flexible eye shield phantom would be applied to fully MC dose calculations and for comparative evaluation with algorithms of existing commercially available TPS.
Conclusions
In this study, flexible dummy shield phantoms for newly designed eye shields, offering a more comfortable fit for patients than existing acrylic dummy shields, were fabricated. In addition, it was possible to acquire artifact-free CT images, which not only facilitates accurate delineation of the OAR and target volume but also indicates its suitability for use in dose calculation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download