Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is a refractory disease that can lead to severe destruction of the jaw. As there is no standard protocol for treating MRONJ, various treatments have been studied. Teriparatide has been used as an adjunct therapy for MRONJ. However, its effectiveness has not been sufficiently demonstrated for use as a standard treatment for MRONJ. This study aimed to demonstrate the efficacy of teriparatide in treating MRONJ by presenting two successfully treated cases. Each patient received teriparatide therapy with surgical intervention. The appropriateness of teriparatide use was evaluated based on the patient’s systemic condition, and the administration of teriparatide was supervised by a physician. Complete resolution of the lesion was observed clinically and radiographically in both patients. The first patient underwent implant placement at the lesion site. Due to its anabolic properties and ability to stimulate bone remodeling, teriparatide is an effective adjunctive pharmacological treatment for bone healing before and after surgery with associated beneficial effects on bone and mucosal healing.
Medication-related osteonecrosis of the jaw (MRONJ) is a rare but refractory condition of the maxillofacial region. MRONJ is defined as an exposed bone or bone that is accessible through an intraoral or extraoral fistula in the maxillofacial region that has persisted for more than eight weeks in patients who are currently being or were previously treated with antiresorptive therapy alone or in combination with immune modulators or antiangiogenic medications and who have no history of radiation therapy to the jaw1. This condition is often associated with pain, infection, purulent secretions, exposed necrotic bone, paresthesia, pathologic fractures, and a low quality of life. Since the first case of MRONJ was described in 20032, various attempts have been made to elucidate its pathophysiological mechanisms; however, these mechanisms remain unclear. Therefore, MRONJ management remains controversial. The interventions used to treat this complex disease are diverse and include conservative, surgical, and adjunctive therapies.
Teriparatide is a recombinant peptide derived from the first 34 amino acids at the N-terminus of the intact human parathyroid hormone3. Parathyroid hormone affects bone metabolism by regulating calcium and phosphorus balance. While continuous administration of parathyroid hormone results in bone resorption, intermittent administration of low doses of parathyroid hormone stimulates bone formation4. Teriparatide has been approved as an anabolic agent for treating osteoporosis in men and postmenopausal women. It stimulates osteoblasts, the cells responsible for bone formation, leading to increased bone mass and improved bone quality5. Teriparatide administration as an adjunctive treatment for MRONJ was first reported by Harper and Fung6 in 2007. Since then, several case reports have documented favorable therapeutic outcomes using teriparatide for MRONJ treatment owing to its osteoanabolic properties that promote bone formation when administered intermittently at low doses7-11.
This case report describes two cases of MRONJ that were successfully treated with teriparatide in conjunction with surgical intervention.
This study was approved by the Institutional Review Board (IRB) of Kyung Hee University Hospital in Gangdong (IRB No. KHNMC 2024-03-021). Due to the retrospective nature of the study, the requirement for written informed consent was waived.
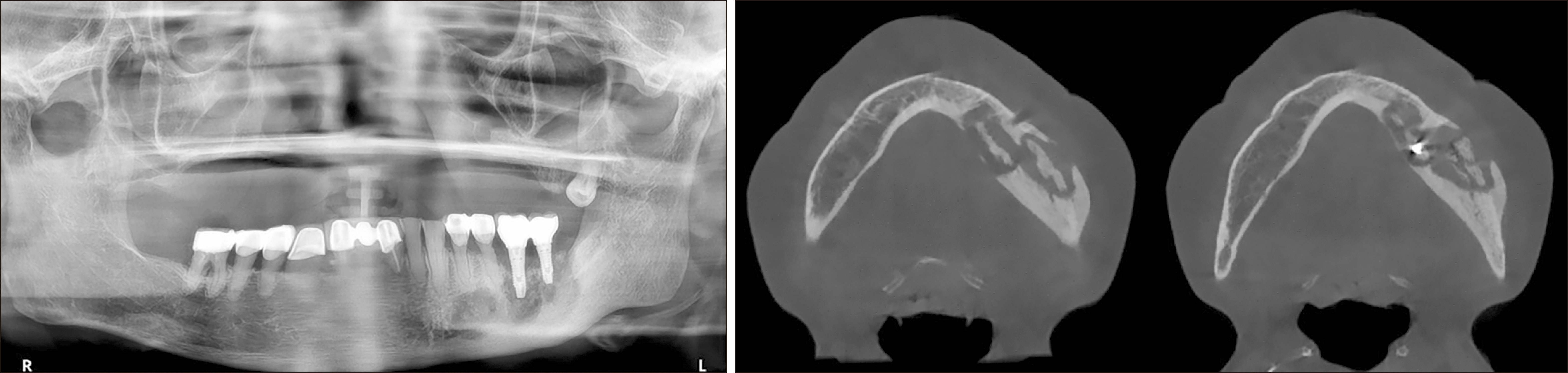
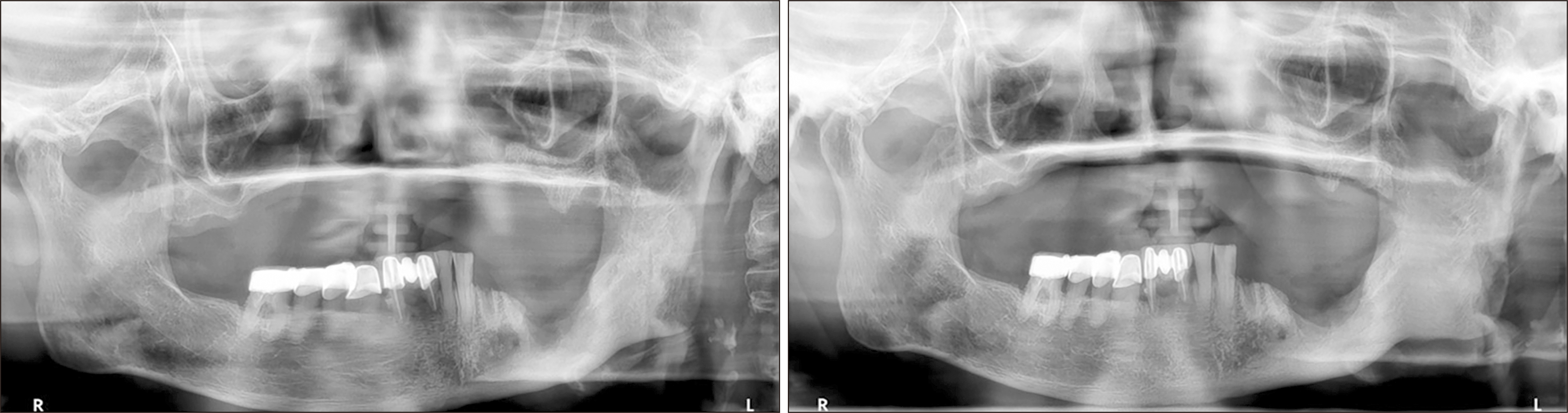
A 78-year-old female with a 5-year history of osteoporosis was referred to our institution for exposed bone with necrotic changes in her left maxillary posterior area 3 months after extraction of the left maxillary first premolar, second premolar, and second molar at a local dental clinic. She had been taking oral bisphosphonate medications for osteoporosis for the previous 5 years but additionally, she had comorbid uncontrolled diabetes mellitus (HbA1c=9.9 mmol/L) and hypertension. The first clinical examination in our institution revealed gingival inflammation and exposed necrotic bone in the left maxillary posterior area. A panoramic radiograph showed the presence of an unhealed socket 3 months after tooth extraction. Panoramic radiography also showed bone sequestrum formation in the left maxillary molar area, which appeared as increased bone density characterized by inhomogeneous mineralization with a peripheral radiolucent rim. Computed tomography (CT) also showed a sequestrum in the left maxillary posterior area and sinus involvement consistent with maxillary sinusitis due to oroantral communication.(Fig. 1) She was diagnosed with stage-3 MRONJ1. Conservative treatment consisting of antibiotics, local irrigation, antimicrobial mouth rinse, and oral hygiene instructions was administered to relieve the inflammatory symptoms. The patient had been off bisphosphonate medication for at least 2 months at the time of the first visit. Since MRONJ is a refractory disease, teriparatide treatment was recommended. After consultation with an endocrinologist, bisphosphonate therapy was discontinued and subcutaneous 20 μg daily teriparatide injection was started. After 7 months, teriparatide dose was reduced to every other day for 9 months after consultation with an endocrinologist. One week after initiation of teriparatide injections, when the inflammatory symptoms subsided, the sequestra in the left maxillary premolar and molar areas were removed under local anesthesia. Inflammatory symptoms including bone exposure resolved, and postoperative panoramic radiograph revealed favorable bone healing 4 months after sequestrectomy. However, maxillary sinusitis persisted on CT.(Fig. 2) The patient was referred to the otolaryngology department for consultation due to persistent chronic maxillary sinusitis with persistent oroantral fistula. Eight months after initiation of teriparatide therapy, an otolaryngologist performed endoscopic sinus surgery with debridement and pus drainage under general anesthesia for persistent chronic maxillary sinusitis. Eleven months after starting teriparatide therapy, complete closure of the oroantral fistula and favorable bone healing were observed, and two implants (CMI IS-II Active, 4.0×10.0 mm; NeoBiotech) were placed at the sites of the left upper first and second premolars.(Fig. 3) After 3 months of osseointegration, the implants were exposed, and healing abutments were placed. The implants exhibited excellent stability, and an implant-supported fixed prosthesis was fabricated with a stable peri-implant region.(Fig. 3)
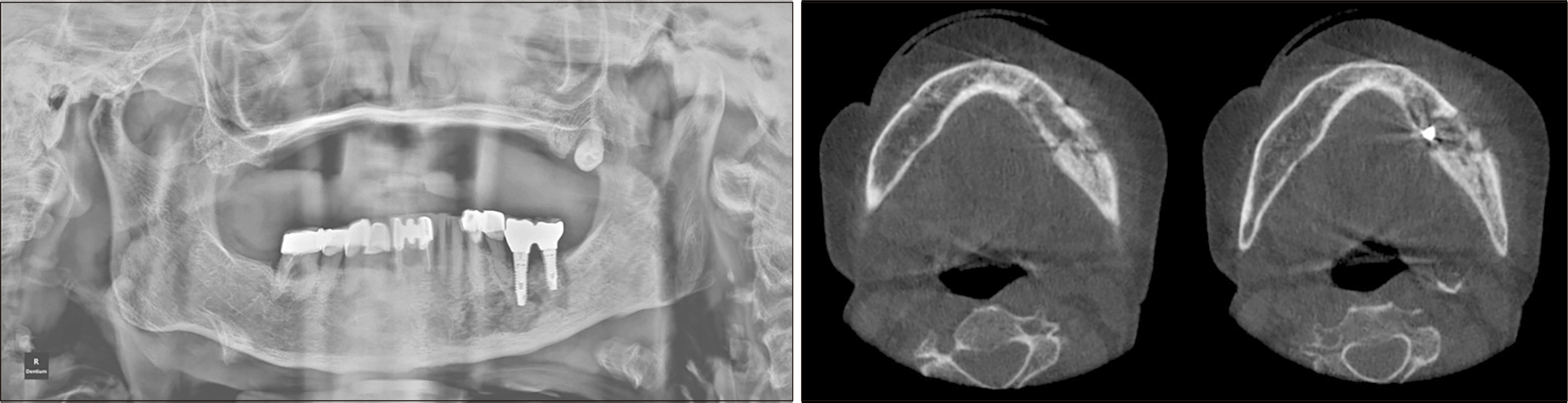
An 81-year-old female with a 4-year history of osteoporosis presented with swelling of the left mandibular angle. She had been receiving antiresorptive therapy for the past 4 years, including subcutaneous denosumab injection for 2 years and oral alendronate for another 2 years. Four months prior, the patient had a history of dental implants placed in the left mandibular first and second molar sites. Clinical findings revealed left submandibular swelling and an intraoral fistula with pus discharge in the buccal gingiva of the dental implants in the left mandibular molar area. Panoramic radiography and CT performed at the initial visit showed extensive bone destruction and sequestration in the left mandibular premolar and molar areas.(Fig. 4) The patient underwent an incision and drainage procedure and was administered intravenous and oral antibiotics. One week after the incision and drainage procedure, an extraoral fistula with purulent discharge developed in the left mandibular area, and she was diagnosed with stage-3 MRONJ1. The patient did not return to the clinic after the symptoms resolved until 2 months later, when she showed recurrent symptoms of left submandibular swelling, pain, and numbness. Owing to extensive osteolysis, teriparatide treatment was recommended due to the increased likelihood of postoperative pathological fractures. After consultation with an endocrinologist, alendronate medication was discontinued; subcutaneous teriparatide injection (20 μg daily) was continued for 8 months and then replaced with another subcutaneous teriparatide injection (20 μg daily) for 4 months. Prior to surgical intervention, conservative treatment consisting of antibiotics, weekly clinical irrigation, antimicrobial mouth rinse, and oral hygiene instructions was implemented. Preoperative panoramic radiography at 7 months and CT at 6 months after the start of teriparatide therapy showed progressive sequestrum separation.(Fig. 5) Surgical intervention, including sequestrum removal, cortication, extraction of the left mandibular premolars, and removal of dental implants in the left mandibular molar area, were performed under general anesthesia. Three weeks after the procedure, the patient presented with complete mucosal coverage of the lesion with no signs of inflammation. Postoperative panoramic radiographs at 2 and 4 months showed resolution of the osteolytic lesion and continued formation of new bone.(Fig. 6)
With a growing global elderly population, the use of antiresorptive or antiangiogenic medications is likely to increase; subsequently, MRONJ incidence is also expected to increase12. The American Association of Oral and Maxillofacial Surgeons classifies MRONJ treatment strategies based on disease stage1. Because the pathogenesis of MRONJ has not yet been established, there is no definitive treatment protocol, and therapeutic strategies range from conservative management to surgical intervention. Additionally, various adjuvant therapies such as teriparatide, recombinant human bone morphogenetic protein-2, hyperbaric oxygen, photobiomodulation, and autologous platelet concentrates have been suggested to promote recovery in patients with MRONJ13.
Teriparatide has emerged as a promising treatment for MRONJ, with potential to activate bone remodeling and stimulate bone formation, promoting bone regeneration. The medication stimulates bone formation by inhibiting osteoblast apoptosis, resulting in an increase in the number of osteoblasts3. Intermittent injections produce an osteoanabolic effect, whereas continuous infusion produces an osteocatabolic effect4. Additionally, repeated administration of teriparatide promotes sequestrum separation, resulting in a clear distinction between necrotic and healthy bones and facilitating sequestrum removal8. Appropriate low-dose intermittent teriparatide combined with other therapies appears to improve the prognosis of patients with MRONJ14. Because of these properties, several studies have reported favorable therapeutic outcomes from intermittent low-doses of teriparatide for treating MRONJ6-11. Among the studies that used teriparatide for treating MRONJ, a 2020 randomized controlled study by Sim et al.11 found a higher rate of resolution, increased bone volume, and reduced bone defect size in the teriparatide (20 μg/day) injection group than the placebo group. Although another report suggests a similar therapeutic effect with either daily or weekly teriparatide administration10, the optimal dose and duration of teriparatide therapy for MRONJ management are yet to be established. Furthermore, the safety and efficacy of teriparatide treatment in patients with MRONJ and various underlying medical conditions are being investigated.
Although the therapeutic effect of teriparatide on MRONJ is promising, several issues need to be addressed. Several factors can contribute to patient reluctance toward teriparatide therapy. These include economic reasons, the inconvenience of daily self-injections, and the burden of frequent clinic visits for high-dose weekly injections9. Adverse side effects of teriparatide therapy include nausea, vomiting, headache, arthralgia, malaise, and renal disfunction15,16. Similar to endogenous parathyroid hormone, teriparatide also affects calcium and phosphate homeostasis through transient increases in serum calcium levels and urinary calcium excretion16,17, which can cause hypercalcemia, calciphylaxis, cutaneous calcification, and urolithiasis. Furthermore, teriparatide-induced hypercalcemia may predispose patients to digoxin digitalis toxicity16.
There is also an increased risk of osteosarcoma associated with teriparatide administration. Teriparatide use presents challenges in certain patient groups due to the potential risk of osteosarcoma, especially in those with open epiphyses (pediatric and young patients), metabolic bone diseases including Paget’s disease, metastasis, a history of skeletal malignancies, prior external beam or implant radiation therapy involving the skeleton, or hereditary disorders16,18. Therefore, teriparatide therapy is not recommended for longer than 2 years over the lifetime to minimize the potential risk of osteosarcoma, and use of teriparatide for longer than two collective years should only be considered if the patient remains at, or has returned to, a high risk of fracture16,18. However, an association between teriparatide therapy and increased osteosarcoma risk has not been identified in humans based on data from clinical trials or post-marketing surveillance16,19.
Unlike bisphosphonates, the decision to discontinue denosumab must be considered with caution because its antiresorptive effect is lost upon discontinuation, which can lead to osteoclast rebound activity characterized by increase in bone turnover markers (BTMs) and a consequent loss of bone mineral density (BMD), with increased fracture risk20,21. Although such discontinuation leads to increased bone turnover and decreased BMD, the underlying mechanisms remain unclear. The primary mechanism proposed is an excessively high remodeling rate that exceeds pretreatment levels. This rapid remodeling is expected to affect trabecular bone more rapidly and significantly than cortical bone, potentially accounting for multiple vertebral fractures22. Therefore, risk-benefit analysis should be performed before deciding to use teriparatide for MRONJ treatment in patients with a history of denosumab.
In these cases, the durations of teriparatide therapy were 16 months and 12 months, respectively. No significant adverse effects of teriparatide therapy were observed. The patient in the first case underwent sequestrectomy one week after the initial teriparatide injection, when the inflammatory symptoms had subsided. Teriparatide therapy was continued until the implant achieved stable attachment at the lesion site. In the second case, teriparatide injections were used to facilitate separation of the sequestrum, allowing its removal and subsequent debridement of the affected area. The optimal time for surgical removal of necrotic bone was determined by CT monitoring. Teriparatide therapy was discontinued after recovery of normal mucosal coverage, with no recurrence thereafter. Surgical intervention was performed in both cases and has been shown effective for treating advanced MRONJ stages23. Surgical treatment for MRONJ includes debridement, curettage, sequestrectomy, and resection, with or without microvascular reconstruction1. However, aggressive surgical interventions such as extensive resection and reconstruction can cause severe disability, especially in elderly patients with impaired wound healing ability and a high risk of comorbidities. Although some studies have used low-dose intermittent daily teriparatide to treat MRONJ without surgery7,8, other studies have shown that patients treated with teriparatide in combination with another therapeutic method were more likely to experience complete resolution of osteonecrosis than those treated with teriparatide alone14. Thus, when treating patients with MRONJ, teriparatide therapy can be effectively used as an adjuvant treatment before or after surgical intervention to promote bone remodeling and sequestrum separation, allowing for a minimally invasive surgical procedure.
In this case report, teriparatide was prescribed in consultation with the Department of Endocrinology and Metabolism at Kyung Hee University Hospital at Gangdong, and the patients were followed regularly by an endocrinologist to ensure that the injections were safely prescribed. Additionally, an antiresorptive agent should be administered after teriparatide therapy because the anabolic effects are rapidly lost after discontinuation24. After termination of teriparatide therapy, our patients were treated with denosumab under the supervision of an endocrinologist. This coordinated approach to MRONJ treatment with a specialized medical department can help determine the appropriateness of teriparatide use based on the patient’s systemic condition, ensure safe teriparatide therapy during MRONJ treatment, and facilitate follow-up treatment of underlying conditions such as osteoporosis.
Due to its non-randomized design and low overall quality of evidence, this case report offers limited insight into the efficacy and long-term effects of teriparatide for MRONJ treatment. The inherent risk of bias in such studies complicates a definitive assessment. Therefore, randomized clinical trials with extended individual cohorts and long-term follow-up are required to ensure the efficacy and safety of teriparatide and to establish a teriparatide therapy protocol for MRONJ management.
This study demonstrated that appropriate, intermittent, low-dose teriparatide application before and after surgical intervention induces favorable resolution for MRONJ management. However, further studies are required to validate teriparatide therapy as an evidence-based approach using a defined protocol in MRONJ management.
Notes
Authors’ Contributions
R.K. has conceived and drafted the manuscript. Y.J. participated in the study design and performed the surgery. Y.J., S.H., J.J., M.L., and Y.L. reviewed the paper. All authors read and approved the final manuscript.
References
1. Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. 2022; American Association of Oral and Maxillofacial Surgeons' position paper on medication-related osteonecrosis of the jaws-2022 update. J Oral Maxillofac Surg. 80:920–43. https://doi.org/10.1016/j.joms.2022.02.008. DOI: 10.1016/j.joms.2022.02.008. PMID: 35300956.

2. Marx RE. 2003; Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 61:1115–7. https://doi.org/10.1016/s0278-2391(03)00720-1. DOI: 10.1016/S0278-2391(03)00720-1. PMID: 12966493.

3. Canalis E. 2018; Management of endocrine disease: novel anabolic treatments for osteoporosis. Eur J Endocrinol. 178:R33–44. https://doi.org/10.1530/eje-17-0920. DOI: 10.1530/EJE-17-0920. PMID: 29113980. PMCID: PMC5819362.

4. Chen T, Wang Y, Hao Z, Hu Y, Li J. 2021; Parathyroid hormone and its related peptides in bone metabolism. Biochem Pharmacol. 192:114669. https://doi.org/10.1016/j.bcp.2021.114669. DOI: 10.1016/j.bcp.2021.114669. PMID: 34224692.

5. de Souza Tolentino E, de Castro TF, Michellon FC, Passoni ACC, Ortega LJA, Iwaki LCV, et al. 2019; Adjuvant therapies in the management of medication-related osteonecrosis of the jaws: systematic review. Head Neck. 41:4209–28. https://doi.org/10.1002/hed.25944. DOI: 10.1002/hed.25944. PMID: 31502752.

6. Harper RP, Fung E. 2007; Resolution of bisphosphonate-associated osteonecrosis of the mandible: possible application for intermittent low-dose parathyroid hormone [rhPTH(1-34)]. J Oral Maxillofac Surg. 65:573–80. https://doi.org/10.1016/j.joms.2006.10.076. DOI: 10.1016/j.joms.2006.10.076. PMID: 17307613.

7. Kim KM, Park W, Oh SY, Kim HJ, Nam W, Lim SK, et al. 2014; Distinctive role of 6-month teriparatide treatment on intractable bisphosphonate-related osteonecrosis of the jaw. Osteoporos Int. 25:1625–32. https://doi.org/10.1007/s00198-014-2622-8. DOI: 10.1007/s00198-014-2622-8. PMID: 24554340.

8. Zushi Y, Takaoka K, Tamaoka J, Ueta M, Noguchi K, Kishimoto H. 2017; Treatment with teriparatide for advanced bisphosphonate-related osteonecrosis of the jaw around dental implants: a case report. Int J Implant Dent. 3:11. https://doi.org/10.1186/s40729-017-0074-6. DOI: 10.1186/s40729-017-0074-6. PMID: 28361376. PMCID: PMC5374080.

9. Choi SY, Yoon D, Kim KM, Kim SJ, Kim HY, Kim JW, et al. 2024; Adjunctive recombinant human parathyroid hormone agents for the treatment of medication-related osteonecrosis of the jaw: a report of three cases. J Korean Assoc Oral Maxillofac Surg. 50:103–9. https://doi.org/10.5125/jkaoms.2024.50.2.103. DOI: 10.5125/jkaoms.2024.50.2.103. PMID: 38693133. PMCID: PMC11063736.

10. Kim KM, Kim S, Hwang H, Kim HY, Kim D, Park JH, et al. 2024; Effects of daily versus weekly teriparatide for medication-related osteonecrosis of the jaw: a case-control study. Oral Dis. 30:3286–95. https://doi.org/10.1111/odi.14801. DOI: 10.1111/odi.14801. PMID: 37927178.

11. Sim IW, Borromeo GL, Tsao C, Hardiman R, Hofman MS, Papatziamos Hjelle C, et al. 2020; Teriparatide promotes bone healing in medication-related osteonecrosis of the jaw: a placebo-controlled, randomized trial. J Clin Oncol. 38:2971–80. https://doi.org/10.1200/jco.19.02192. DOI: 10.1200/JCO.19.02192. PMID: 32614699.

12. Adami G, Fassio A, Gatti D, Viapiana O, Benini C, Danila MI, et al. 2022; Osteoporosis in 10 years time: a glimpse into the future of osteoporosis. Ther Adv Musculoskelet Dis. 14:1759720X221083541. https://doi.org/10.1177/1759720x221083541. DOI: 10.1177/1759720X221083541. PMID: 35342458. PMCID: PMC8941690.

13. Shim GJ, Ohe JY, Yoon YJ, Kwon YD, Kim DY. 2022; Current trends in adjuvant therapies for medication-related osteonecrosis of the jaw. Appl Sci. 12:4035. https://doi.org/10.3390/app12084035. DOI: 10.3390/app12084035.

14. Dos Santos Ferreira L, Abreu LG, Calderipe CB, Martins MD, Schuch LF, Vasconcelos ACU. 2021; Is teriparatide therapy effective for medication-related osteonecrosis of the jaw? A systematic review and meta-analysis. Osteoporos Int. 32:2449–59. https://doi.org/10.1007/s00198-021-06078-z. DOI: 10.1007/s00198-021-06078-z. PMID: 34331067.

15. Anabtawi M, Tweedale H, Mahmood H. 2021; The role, efficacy and outcome measures for teriparatide use in the management of medication-related osteonecrosis of the jaw. Int J Oral Maxillofac Surg. 50:501–10. https://doi.org/10.1016/j.ijom.2020.07.021. DOI: 10.1016/j.ijom.2020.07.021. PMID: 32800674.

16. Eli Lilly and Company. 2024. FORTEO (teriparatide injection) [Internet]. U.S. Food and Drug Administration;Silver Spring (MD): Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021318Orig1s056lbl.pdf. cited 2024 May 31.
17. Mohammad Ismail SH, Simatherai D, Peraba P, Bee BC, Wong HS, Yakob S. 2023; Teriparatide use in the treatment of severe hypocalcemia after kidney transplantation: a case report. Kidney Transplant Transplant Immunol. 8(3 Suppl):S395. https://doi.org/10.1016/j.ekir.2023.02.886. DOI: 10.1016/j.ekir.2023.02.886.

18. Krege JH, Gilsenan AW, Komacko JL, Kellier-Steele N. 2022; Teriparatide and osteosarcoma risk: history, science, elimination of boxed warning, and other label updates. JBMR Plus. 6:e10665. https://doi.org/10.1002/jbm4.10665. DOI: 10.1002/jbm4.10665. PMID: 36111201. PMCID: PMC9465003.

19. Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH, et al. 2012; The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res. 27:2429–37. https://doi.org/10.1002/jbmr.1768. DOI: 10.1002/jbmr.1768. PMID: 22991313. PMCID: PMC3546381.

20. Burckhardt P, Faouzi M, Buclin T, Lamy O. The Swiss Denosumab Study Group. 2021; Fractures after denosumab discontinuation: a retrospective study of 797 cases. J Bone Miner Res. 36:1717–28. https://doi.org/10.1002/jbmr.4335. DOI: 10.1002/jbmr.4335. PMID: 34009703. PMCID: PMC8518625.

21. Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, et al. 2015; Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 386:1147–55. https://doi.org/10.1016/s0140-6736(15)61120-5. DOI: 10.1016/S0140-6736(15)61120-5. PMID: 26144908.

22. Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. 2017; Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 32:1291–6. https://doi.org/10.1002/jbmr.3110. DOI: 10.1002/jbmr.3110. PMID: 28240371.

23. Ristow O, Rückschloß T, Müller M, Berger M, Kargus S, Pautke C, et al. 2019; Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J Craniomaxillofac Surg. 47:491–9. https://doi.org/10.1016/j.jcms.2018.12.014. DOI: 10.1016/j.jcms.2018.12.014. PMID: 30642734.

24. Black DM, Rosen CJ. 2016; Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 374:254–62. https://doi.org/10.1056/nejmcp1513724. DOI: 10.1056/NEJMcp1513724. PMID: 26789873.

Fig. 1
Panoramic radiograph on initial visit (upper left). Axial computed tomography (CT) view on initial visit (upper right). Coronal CT view on initial visit (lower left). Sagittal computed tomography CT view on initial visit (lower right).
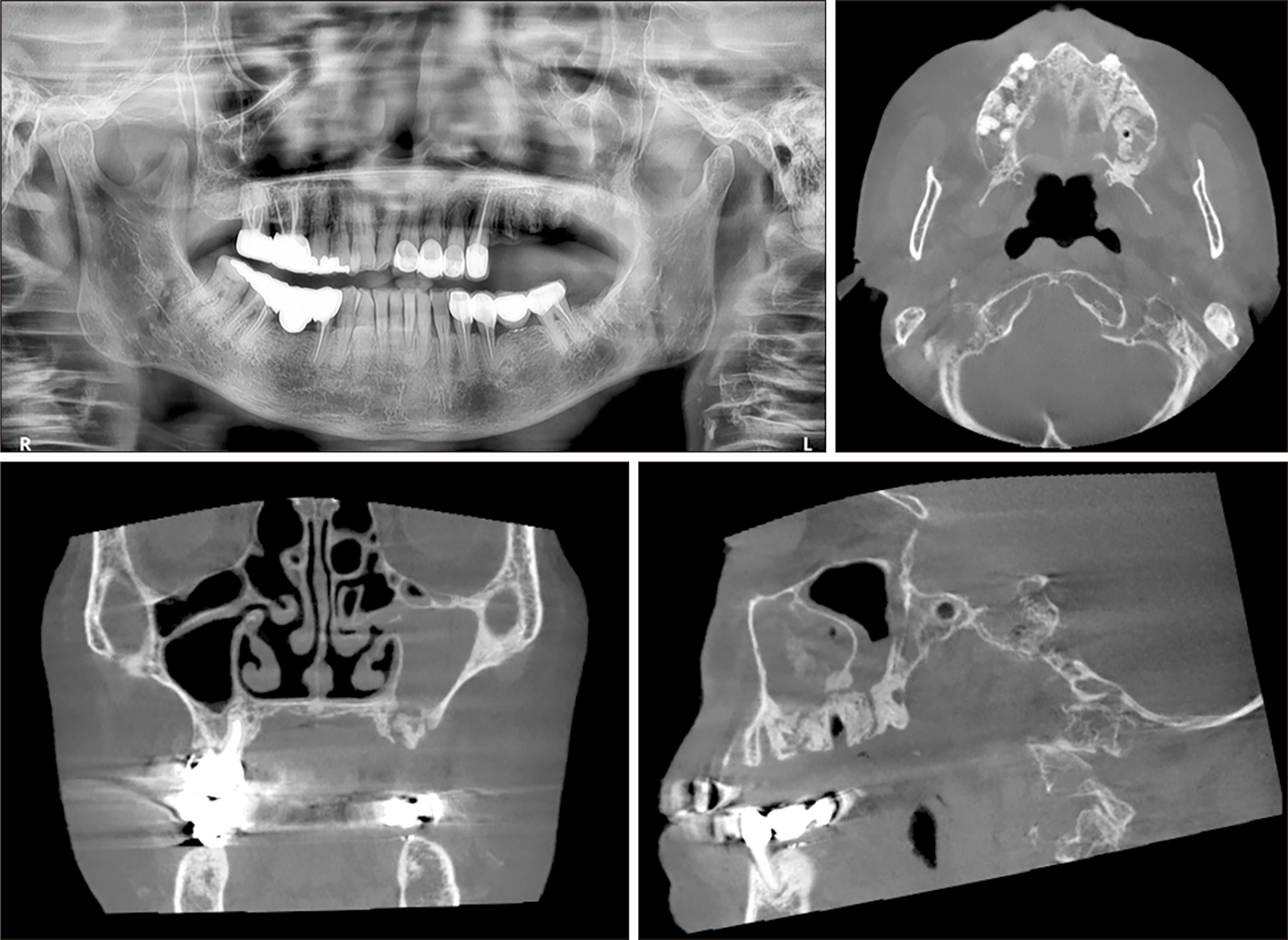
Fig. 2
Postoperative panoramic radiograph at four months after sequestrectomy (upper left). Postoperative computed tomography axial, coronal, and sagittal views at 4 months after sequestrectomy (upper right, lower left, lower right, respectively) show persistent maxillary haziness.
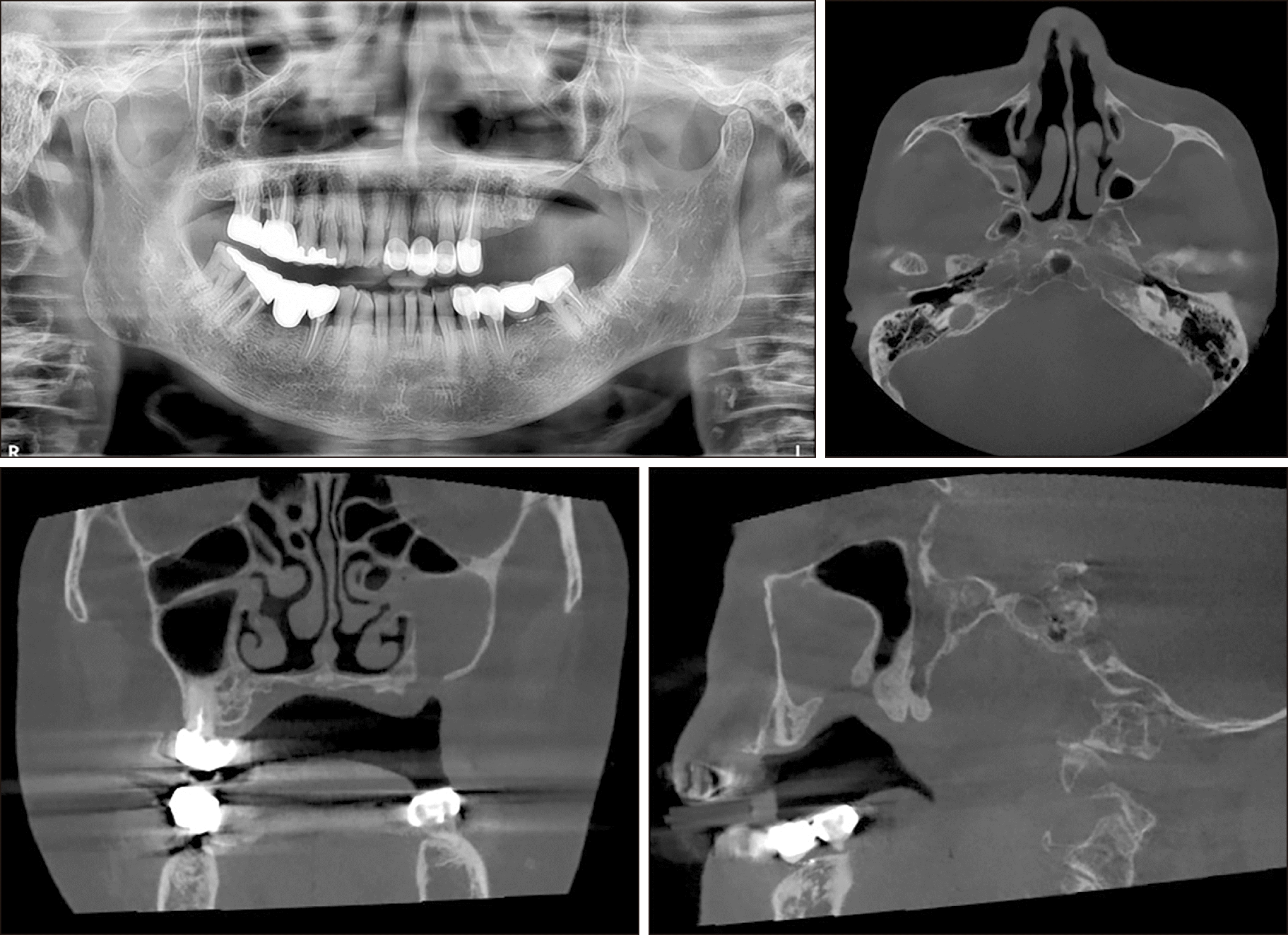
Fig. 3
Panoramic radiograph after implantation at the lesion site (left). Intraoral radiograph 4 months after implantation (right).
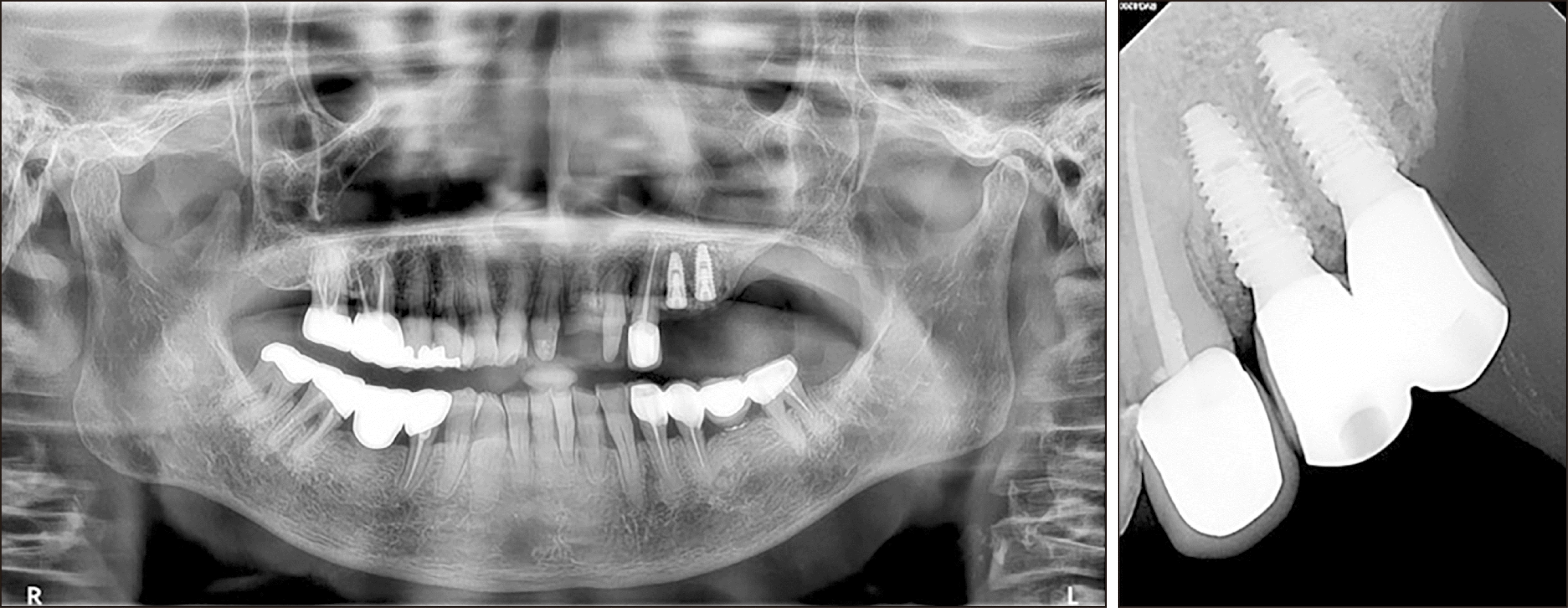
Fig. 4
Panoramic radiograph on initial visit (left). Axial computed tomography view on initial visit (right).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download