This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Dextrose prolotherapy is one of the most promising minimally invasive interventions for temporomandibular disorder (TMD), particularly in refractory cases where other conservative treatments have failed. The purpose of this study was to demonstrate the efficacy of a new treatment, temporomandibular joint (TMJ) prolotherapy, in patients with TMD to alleviate symptoms.
Materials and Methods
A retrospective analysis was conducted on TMD patients with chronic pain who did not respond to conventional treatments. TMJ prolotherapy was performed using hypertonic dextrose in the TMJ area, targeting the retrodiscal attachment tissue, anterior disc attachment tissue, lateral capsule, origin of the masseter muscle, and the stylomandibular ligament. Pain or discomfort intensity in the TMJ was evaluated using a numerical rating scale (NRS). Maximum mouth opening and subjective satisfaction were also analyzed.
Results
Nineteen patients (6 males, 13 females, average age 43 years) participated in this study. All patients experienced pain improvement with a maximum of three prolotherapy sessions. The initial mean NRS was 5.7, which ultimately decreased to a final mean TMJ discomfort score of 1.7 post-intervention. The patients’ maximum mouth opening increased from an initial 34.5 mm to 38.8 mm, and they reported positive satisfaction with the prolotherapy treatment. The clinical outcomes were positive regardless of main origin of TMD symptoms.
Conclusion
Hypertonic TMJ prolotherapy is an effective minimally invasive intervention for TMJ disorders with chronic pain.
Keywords: Prolotherapy, Temporomandibular joint disorders, Chronic pain, Minimally invasive surgical procedures
I. Introduction
The temporomandibular joint (TMJ) has unique characteristics, including the ability to move in three planes, its role as a load-bearing joint, and its direct relationship with the dentition. Therefore, orthopedic principles typically used for managing other acutely injured joints may not be applicable to the TMJ
1. The management of temporomandibular disorder (TMD) is gaining more attention in oral and maxillofacial surgery due to the increasing number of affected patients. In recent decades, the emergence of arthroscopy and arthrocentesis has significantly shifted the primary treatment approach for TMD from surgical to non-surgical, minimally invasive interventions. Primary conservative treatments include medications (nonsteroidal anti-inflammatory drugs [NSAIDs]) for anti-inflammation and pain control, occlusal splint therapy, and physiotherapy
2. If conservative treatment fails, surgical treatment is considered. Before performing open joint surgery, many clinicians attempt minimally invasive interventions such as botulinum toxin A (BTX) injections into the associated muscles, steroid or hyaluronic acid injections into the joint space, arthrocentesis, or arthroscopy. BTX muscle injections, which inhibit muscle hyperactivity, as well as superior joint injections and arthrocentesis, which lavage inflammatory substances in the joint cavity, are effective in correcting joint function and reducing pain
3. However, the causes of TMD can also include inflammatory conditions and chronic pain of various structures such as the articular disc, retrodiscal tissue, TMJ-related ligaments and capsule, and tendons.
While various treatment options exist for TMD, one alternative gaining attention is prolotherapy, defined as “rehabilitating an incompetent structure such as a ligament or tendon by stimulating cellular proliferation”
3. Prolotherapy, also known as “regeneration injection therapy,” is a non-surgical treatment method that aims to promote tissue repair and stimulate the body’s natural healing process
4. It involves injecting a solution; often a mixture of dextrose, anesthetics, and saline; into the affected area. These injections aim to initiate a controlled inflammatory response, triggering the body to repair damaged tissues and strengthen the joint. When applied to the TMJ, prolotherapy targets the ligaments, tendons, and other connective tissues supporting the joint
4. These structures can weaken or become damaged due to trauma, repetitive strain, or degenerative conditions, contributing to TMD symptoms. By stimulating the healing response, prolotherapy aims to strengthen the affected tissues, reduce pain, and improve joint function.
Although the exact cause of chronic TMD remains controversial, a dextrose solution greater than 10% can stimulate the proliferation of fibroblasts through both inflammatory and non-inflammatory mechanisms. This strengthens the TMJ-related connective tissue and significantly reduces pain levels in chronic TMD patients
5. Dextrose is easily accessible, highly safe, and economical, decreasing the patient burden of TMJ prolotherapy. Thus, it may be beneficial for patients with early-stage TMD, those with refractory TMD who have had limited success with other treatments, and patients who are unwilling or unable to undergo TMJ surgery
6. This study aims to evaluate the clinical efficacy of TMJ prolotherapy in chronic TMD patients who do not respond to conservative and interventional treatments.
II. Materials and Methods
1. Patients
This retrospective study was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-2402-882-101) and included TMD patients who received hypertonic dextrose prolotherapy treatment in the TMJ area from January 2022 to December 2023. The inclusion criteria were: (1) patients with TMD-related pain not alleviated by conventional treatments (including splint therapy, botulinum injection into the masseter or temporalis muscle, dexamethasone or hyaluronic acid injection into the TMJ superior joint space, and TMJ arthroscopy-arthrocentesis); (2) patients treated with TMJ prolotherapy performed by an expert operator (Y.K.K. with over 35 years of experience) and; (3) patients who voluntarily responded to the Numerical Rating Scale (NRS) questionnaire regarding TMJ-related pain. The exclusion criteria were: (1) patients with diabetes mellitus; (2) those with TMJ tumors and severe condyle ankyloses; (3) those with a history of TMJ open surgery; (4) patients taking NSAIDs or steroids for systemic diseases; and (5) patients requiring psychiatric treatment.
The diagnosis of TMJ disorders was based on the criteria established by the Japanese Society for the Temporomandibular Joint in 2001. The five categories are: type I - Masticatory muscle disorder; type II - Capsule-ligament disorder; type III - Disc disorder; type IV - Degenerative joint disease, osteoarthritis, or osteoarthrosis; and type V - Psychiatric issues not included in types I-IV. Patients primarily presenting symptoms related to soft tissues were categorized into group A (types I-III), and those with type IV were categorized into group B.
2. Treatment protocol
Prior to prolotherapy injection, an auriculotemporal nerve block was administered using 2 mL of 2% lidocaine without vasoconstrictor
7, and topical anesthesia was achieved by applying EMLA cream (AstraZeneca) to the skin. Based on previous studies on the concentration of dextrose injection, the prolotherapy solution was prepared by combining dextrose (20%), lidocaine (4%), and saline in a volume ratio of 2:1:1, resulting in a 10% dextrose solution
8,9. A quantity ensuring that 1 cc of the mixed hypertonic dextrose solution was injected at each point was administered. The anatomical points targeted for TMJ prolotherapy included the retrodiscal attachment tissue, anterior disc attachment tissue, superior portion of the lateral capsule, inferior portion of the lateral capsule, origin of the masseter muscle (zygomatic arch), and insertion point of the stylomandibular ligament. A 26-gauge 22-mm needle was used for the prolotherapy injection and for the auriculotemporal nerve block. For pain control, Ultracet ER semi-tab (Janssen Korea) 325/37.5 mg was prescribed for 2 days. Other interventions were discontinued during prolotherapy treatment and the follow-up period.
3. Number of interventions
The prolotherapy injections were administrated with a 2-3 week interval. The decision to continue or discontinue the treatment was based on patient compliance and positive outcomes.
4. Clinical outcome
The extent of TMJ disorder was assessed using a 0 to 10 NRS, where a score of 0 indicated “no pain or dysfunction,” while a score of 10 indicated “the most severe pain or dysfunction imaginable.” Dysfunction symptoms included pain in the TMJ area, mouth opening limitation (mouth opening <35 mm), hypermobility of the jaw (mouth opening >50 mm), and TMJ sound (clicking, crepitus, popping), all recorded according to the patient’s subjective degree of discomfort. The NRS is frequently used to measure the alleviation of discomfort among patients who underwent therapeutic interventions for TMD
10. These symptoms were periodically measured at each session to compare the difference before and after prolotherapy treatment. The treatment efficacy was assessed 2 to 3 weeks after prolotherapy treatment. According to the TMD type, the clinical outcomes were statistically analyzed using the Mann–Whitney U test. The data are presented as the mean±standard deviation, and statistical analysis was performed using SPSS Statistics for Windows ver. 27.0 (IBM).
III. Results
This study included 19 patients (6 males, 13 females, average age 43 years, ranging from 18 to 74 years;
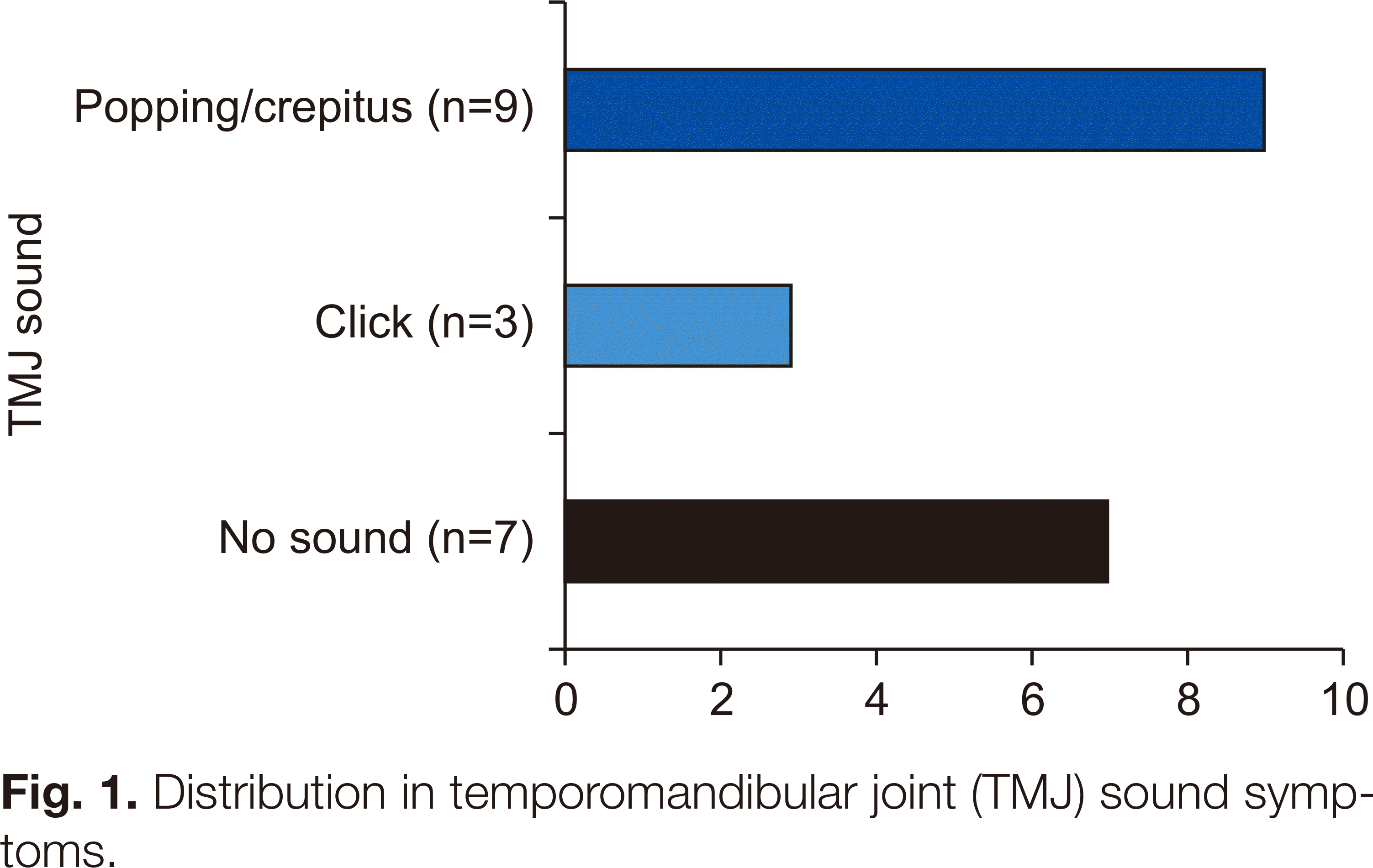
Table 1), 11 of whom had parafunctional habits such as bruxism or clenching. Nine patients had popping or crepitus, three had clicking, and seven had no disc problems.(
Fig. 1) Prolotherapy was administered up to three times. Thirteen patients (68.4%) received three prolotherapy sessions, two patients (10.5%) experienced symptom improvement after two sessions, and four patients (21.1%) experienced symptom improvement after one session.(
Table 1)
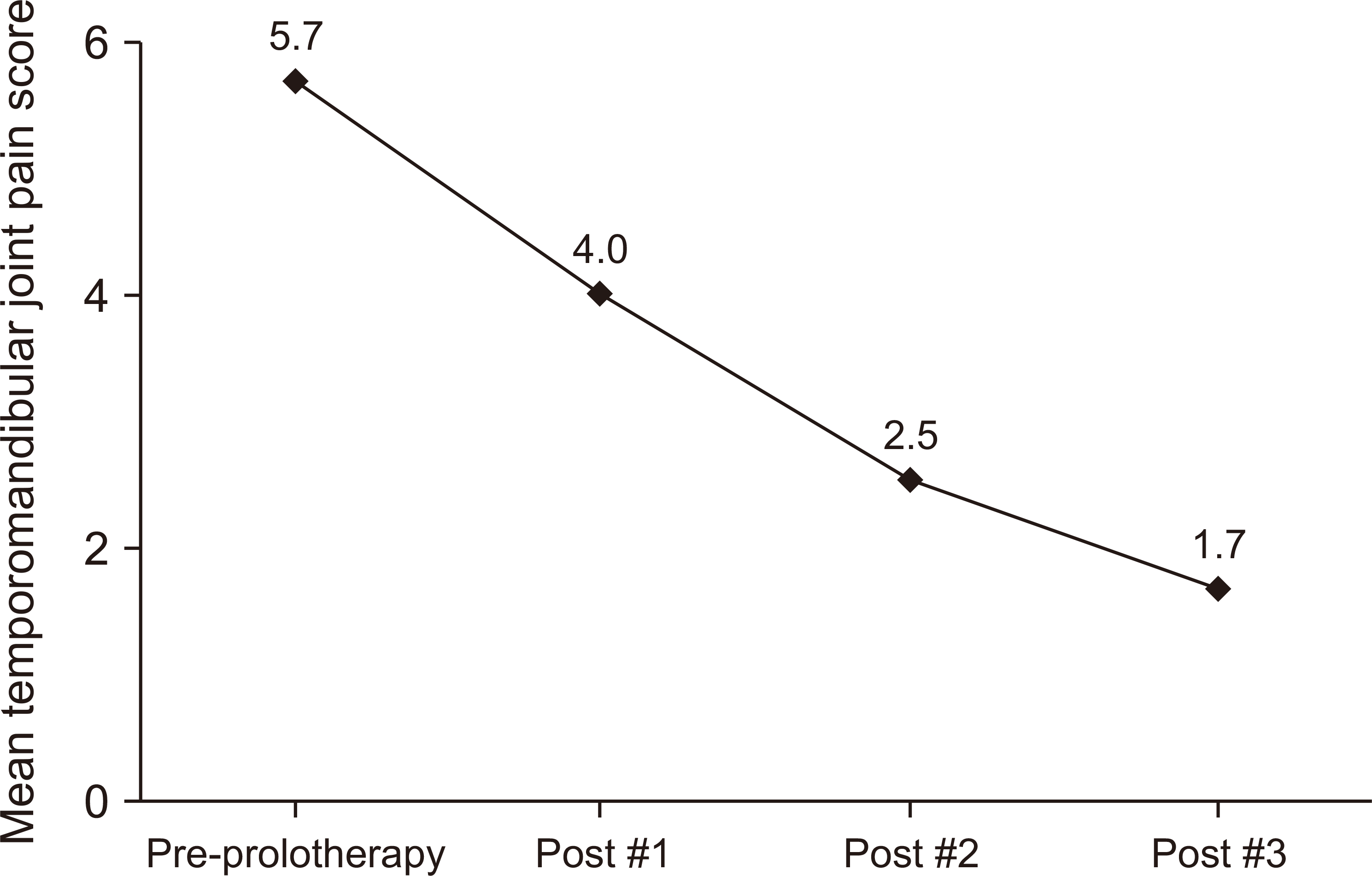
In the cohort that underwent a series of three prolotherapy sessions, the initial mean TMJ discomfort value (NRS) was recorded at 5.7 (n=19). Among those who received all three sessions, the mean NRS score decreased progressively, with a mean score of 4.0 (n=19) after the first session, 2.5 (n=15) after the second session, and ultimately reaching a final mean score of 1.7 (n=13) upon completion.(
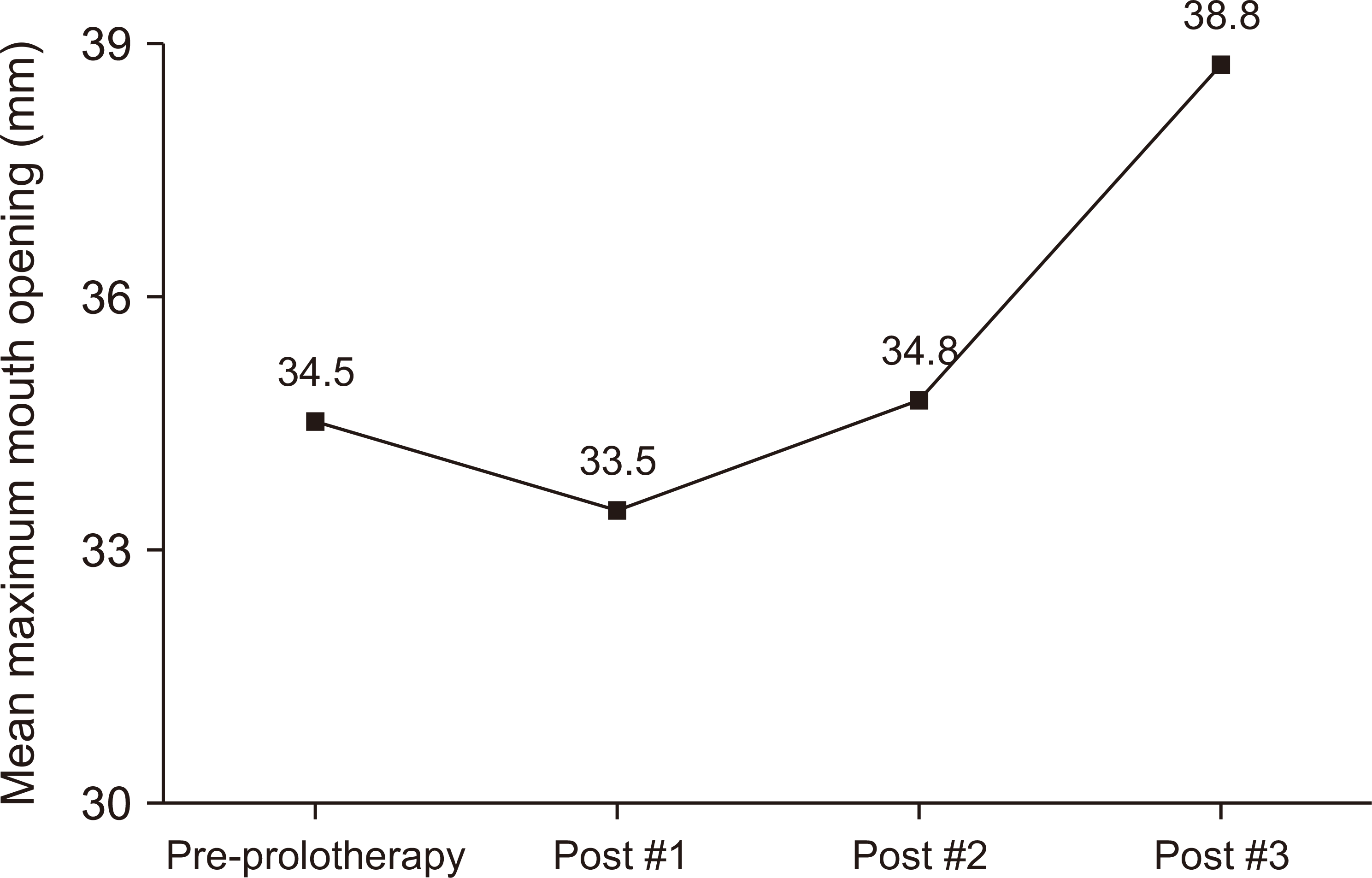
Fig. 2) The mean maximum mouth opening (MMO) tended to decrease after the first prolotherapy session (34.5 mm to 33.5 mm) but gradually increased in subsequent sessions (34.8 mm to 38.8 mm).(
Fig. 3) Overall, the satisfaction of TMD patients after prolotherapy treatment was positive.(
Fig. 4) No serious complications were observed in any patients who received prolotherapy injections. Minor complications immediately following prolotherapy included mild pain at the injection sites and temporary ipsilateral facial nerve weakness, both of which subsided within 2 to 3 hours.
To investigate the effectiveness of prolotherapy in patients with bone-related symptoms such as arthritis, group A included 10 patients diagnosed with TMD type I, II, or III, and group B was nine patients with type IV.(
Fig. 5) Regardless of the main cause of the symptoms, both pain scores and MMO improved after prolotherapy treatment, and both groups showed satisfactory outcomes.(
Table 2)
IV. Discussion
We hypothesized that prolotherapy would be effective in reducing pain for chronic TMD patients who do not respond to conventional conservative treatments, including traditional therapies targeting the masticatory muscles and superior joint space. The clinical outcome was recorded using an NRS and relied on subjective patient opinion. Symptom relief was not specified according to type such as pain, limitation of opening, TMJ sound (clicking, crepitus, popping), or hypermobility of the jaw but was simplified as overall change in level of discomfort. The objective of this study was to substantiate the clinical efficacy of an alternative conservative treatment approach for patients with TMD-related chronic pain. Prolotherapy on the TMJ was effective in decreasing pain and discomfort in the TMJ area, and it also showed an additional effect in improving maximum opening regardless of main origin of symptoms.
Prolotherapy has been recognized since the 1930s for its efficacy in enhancing tendon, ligament, and joint stabilization
4. Prolotherapy is currently being applied in the medical field across diverse anatomical joints, including the knee, finger and lumbar region, yielding favorable outcomes
11-13. The mechanism behind prolotherapy is not yet fully understood. The treatment first causes inflammation at the site of administration, initiating the inflammatory cascade
14. Fibroblasts and endothelial cells mobilize toward the site of injury, promoting neovascularization and cellular proliferation and facilitating collagen deposition. Dextrose administration triggers the synthesis of crucial growth factors, including platelet-derived growth factor, transforming growth factor-beta, connective tissue growth factor, and epidermal growth factor, which are all essential for tissue repair
15. This dynamic tissue remodeling process persists within tendon, ligament, and cartilage tissues over the course of several weeks to a few months. Hauser reported that TMJ prolotherapy could be recommended for patients with refractory TMDs or for those who achieved only limited success with other non-invasive treatments such as oral appliances or medication and for those who are unable or unwilling to receive open surgery
16.
According to Mustafa et al.
9, no significant differences in efficacy were shown for different concentrations of dextrose solution used in prolotherapy injections for TMD. Hakala and Ledermann
17 asserted that “the precise concentration of dextrose is not critical as long as it is strongly hypertonic and causes adequate cell wall lysis to attract fibroblasts and begin the regenerative process.” Therefore, a 10% dextrose solution was used instead of a higher concentration as in other notable TMJ prolotherapy studies
8,10,17. As long as the solution is hypertonic, the prolotherapy treatment initiates the regenerative process by causing cell wall lysis and fibroblast activation
17. However, it is important to avoid prescribing NSAIDs because they will prevent the controlled inflammatory response desired in prolotherapy treatment. Though dextrose solution is considered harmless, patients with uncontrolled or severe diabetes mellitus should be advised against hypertonic dextrose prolotherapy. Aspiration to prevent the inadvertent injection of the prolotherapy solution into blood vessels should always be emphasized.
TMJ prolotherapy is a relatively simple and safe injection procedure for any trained oral maxillofacial surgeon. However, given the various anatomical structures surrounding the TMJ, accurate injection of the dextrose solution into the targeted tissue and due consideration of anatomical variance should always be noted to achieve the most effective results
7. In our experience, patients should be distinguished into two groups for effective prolotherapy treatment. First, patients with TMD-related trismus, muscle and joint pain, or TMJ clicking are categorized as group A prolotherapy. This category of TMD should be treated to limit masseter muscle function and regenerate the damaged ligament tissues attached to the TMJ disc. Therefore, these patients should receive injection into the retrodiscal attachment tissue, anterior disc attachment tissue, superior portion of the lateral capsule, and inferior portion of the lateral capsule. If symptoms persist, the origin of the masseter muscle located at the zygomatic arch can be an additional point for injection. Second, patients with bone-related problems are categorized as belonging to group B, which comprised patients with symptoms such as recurrent TMJ avulsion, hypermobility, or TMJ popping sounds. These patients should also receive injections at the insertion point of the stylomandibular ligament, which functions to limit excessive jaw protrusion. Further studies are needed to determine the most optimized prolotherapy injection points for the various TMD symptoms.
V. Conclusion
TMJ prolotherapy using hypertonic dextrose is a promising non-surgical treatment for chronic pain related with TMD, showing significant reductions in pain and improvements in joint function. Further research is needed to optimize injection points and tailor treatments to specific TMD symptoms for enhanced efficacy.




 PDF
PDF Citation
Citation Print
Print



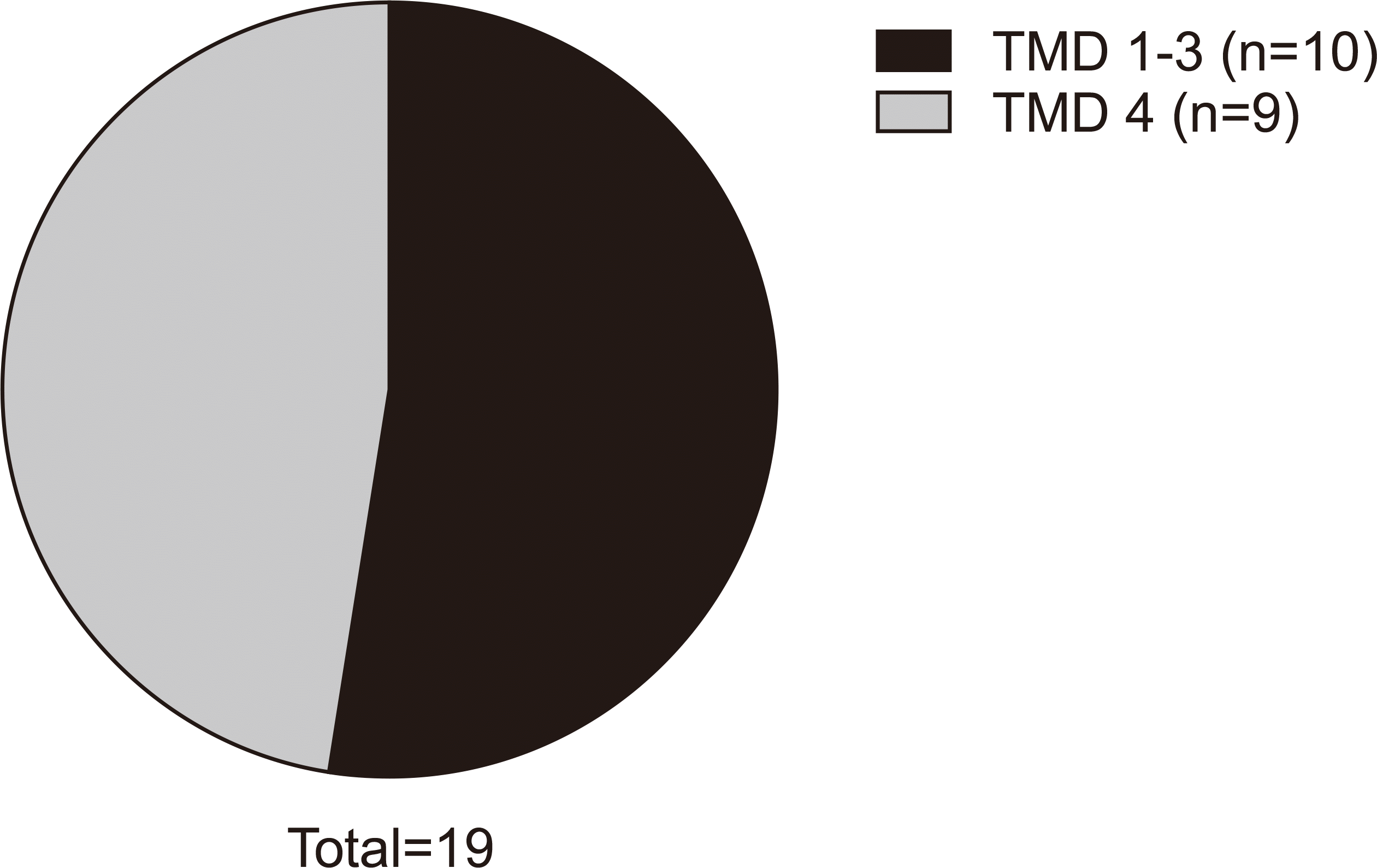
 XML Download
XML Download