This article has been
cited by other articles in ScienceCentral.
Abstract
Septic embolism and stroke are serious complications in patients with sepsis and often necessitate urgent surgical intervention to control the source of infection. A 69-year-old man presented with severe pain in his back and left thigh. MRI revealed extensive posterior epidural or subdural abscesses extending from the cervical to the lumbar level, as well as an abscess in the iliopsoas muscle. The patient underwent urgent drainage of the abscesses and decompression of the lumbar spine. Postoperatively, he developed sudden-onset atrial fibrillation and altered mental status. Brain CT showed multiple embolic infarctions. His condition deteriorated due to persistent infection, leading to disseminated intravascular coagulation, acute kidney injury, and septic shock. This case highlights the risk of cerebral embolism and hemorrhagic complications in patients with sepsis who undergo surgery. Early recognition of individuals at high risk and comprehensive perioperative management are critical to reducing the likelihood of such complications.
Keywords: Embolism, Epidural abscess, Intracranial embolism, Multiple organ failure, Sepsis
Introduction
Patients with sepsis or septic shock may require preoperative optimization. These individuals often need urgent surgical intervention to control the source of infection. The preoperative assessment should prioritize the identification and management of organ dysfunction [
1]. Septic cerebral emboli can also lead to vessel wall infection and potentially result in stroke, particularly in patients with severe sepsis [
2].
Septic embolism occurs when a blood clot containing bacteria, known as a septic embolus, obstructs a blood vessel [
3]. This embolus originates from an infectious site within the body, detaches, and travels through the bloodstream, eventually blocking small vessels. The origin of septic emboli depends on the anatomic location of the infection and the associated vascular structures. Infective endocarditis, for instance, is a well-known and frequent source of septic emboli. Risk factors for these emboli include intravenous drug use and the presence of an indwelling vascular catheter [
4].
In the present case, the primary objective was to provide safe, optimal care for a critically ill patient with sepsis, ensuring that source control surgery could be performed with minimal risk and maximal benefit. This situation presents substantial challenges regarding perioperative management, especially in cases of septic shock. The patient in this report had spinal abscesses extending from the cervical to the lumbar level, which led to the development of cerebral embolism following lumbar spine surgery.
Case presentation
Ethics statement
The patient provided informed consent for publication of the images.
Patient information
A 69-year-old man, measuring 176 cm and weighing 72 kg, presented with severe back pain and radiating pain in his left thigh. His medical history included hypertension, dyslipidemia, and previous lumbar injection.
Clinical findings
Upon physical examination, the patient’s vital signs were unstable, with a blood pressure (BP) of 95/55 mmHg, a heart rate (HR) of 110–115 beats per minute, and a body temperature (BT) of 38.0℃. An ECG revealed sinus tachycardia. Chest radiography appeared normal, with no active lesions evident.
Diagnostic assessment
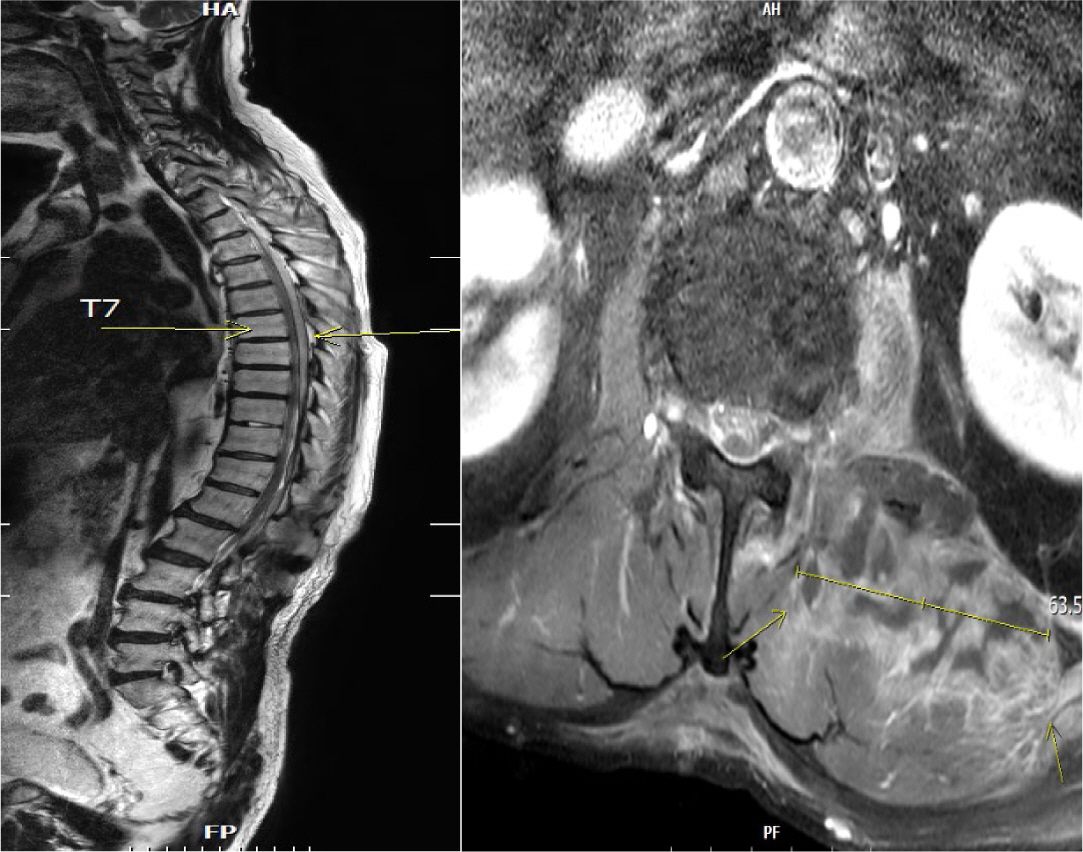
MRI of the spine revealed a posterior epidural or subdural abscess extending from the cervical to the lumbar level, a fluid collection or abscess in the left iliopsoas muscle approximately 7.6 cm in height (
Fig. 1), and intradural disc herniations at the L3–L4 and L4–L5 levels.
Fig. 1.
Spine MRI revealed a posterior epidural or subdural abscess extending from the cervical to the lumbar level. Additionally, an abscess approximately 7.6 cm in height was identified in the left iliopsoas muscle.
Laboratory findings indicated an elevated C-reactive protein level of 41.5 mg/dL, a white blood cell count of over 12,000/µL, and a decreased platelet count of 128,000/µL. Given the suspicion of infection, the patient was promptly started on empiric antibiotic therapy with vancomycin and ceftriaxone, and preparations were made for abscess drainage and L3–L5 posterior decompression.
Upon arrival in the operating room, the patient’s BP was 117/80 mmHg, an ECG indicated sinus tachycardia with an HR of 135 beats per minute, and the measured BT was 38.4℃. For the induction of general anesthesia, 50 mg of rocuronium was administered. Anesthesia was maintained with total intravenous anesthesia using a target-controlled infusion of propofol (2.3–3.0 ng/mL) and remifentanil. The patient was ventilated with a mixture of oxygen and air.
Therapeutic intervention and timeline
Intraoperatively, the patient’s HR remained high (133–145 beats/min), and his BT gradually increased to 38.8℃. These changes are attributed to exacerbation of the patient’s septic condition. Acetaminophen and intravenous fluids were administered to manage the elevated BT. Additionally, the use of heating circuits and body warmers was discontinued to avoid further increases in temperature. Following intravenous hydration, the patient’s HR decreased to 110–120 beats per minute, and his BT stabilized at 37.0℃–37.5℃. Intraoperatively, BP was maintained between 90–105 systolic and 60–70 diastolic mmHg. The fraction of inspired oxygen was set at 0.5, and the patient’s arterial partial pressure of oxygen was approximately 105 mmHg. One hour into surgery, the estimated blood loss exceeded 500 mL while the hemoglobin level fell below 8 g/dL, prompting the transfusion of 2 units of packed red blood cells. Subsequently, the patient’s HR increased to 130–140 beats per minute, and his mean arterial pressure (MAP) dropped to 60–70 mmHg. In response, intravenous fluids and norepinephrine were administered at a rate of 0.05 µg/kg/min to maintain a MAP of at least 70 mmHg. The total duration of anesthesia was 170 minutes, after which the patient was transferred to the post-anesthetic care unit.
In the post-anesthetic care unit, ECG and arterial line monitoring were performed. The patient’s HR was 115–120 beats per minute, and he was mentally alert. However, after 10 minutes, atrial fibrillation was abruptly detected on the ECG, and the patient began to show signs of drowsiness. Subsequently, he became unresponsive and exhibited a pinpoint pupil reflex, necessitating an immediate neurology consultation. To manage the atrial fibrillation, diltiazem was administered for rate control, and norepinephrine was given continuously as the MAP dropped to 65–75 mmHg. Considering the clinical situation, brain CT was deemed necessary and was performed in the CT room. The patient was then transferred to the intensive care unit.
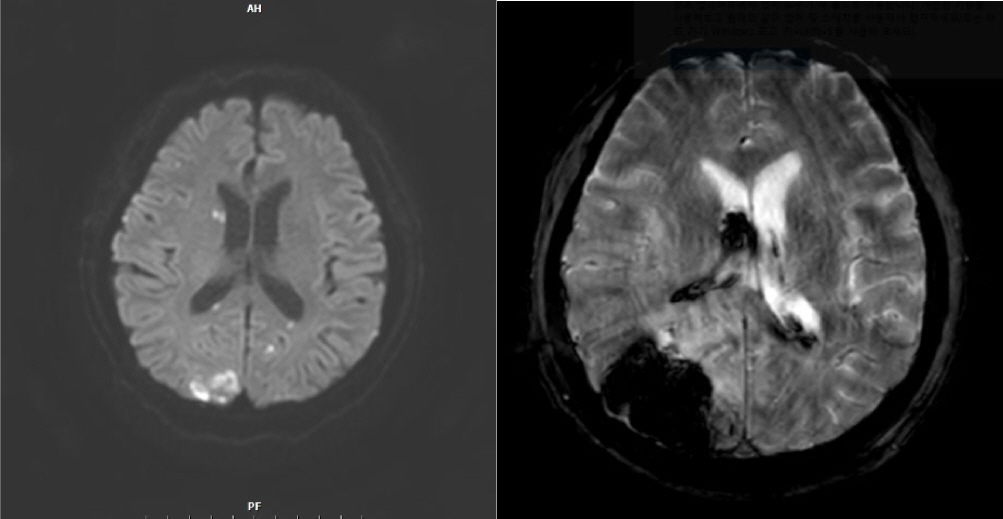
The brain CT scan revealed multiple embolic infarctions (
Fig. 2). However, neurology consultation determined that these infarctions were not the primary cause of the patient’s altered mental status. Consequently, aspirin therapy was initiated. To further evaluate the persistent atrial fibrillation and the possibility of infective endocarditis, transthoracic echocardiography and coronary angiography were performed. These tests indicated stress-induced cardiomyopathy, but no cardiac vegetation was detected.
Fig. 2.
Brain CT revealed multiple bilateral infarctions. On postoperative day 8, the patient developed acute intraventricular hemorrhage in the right lateral ventricle, along with a 4.5-cm intracerebral hemorrhage in the right occipital lobe.
Follow-up and outcomes
On postoperative day (POD) 8, the patient developed acute intraventricular hemorrhage in the right lateral ventricle and intracerebral hemorrhage, measuring 4.5 cm in diameter, in the right occipital lobe. This was likely due to septic thrombosis related to aspirin use or coagulopathy associated with septic shock. Consequently, the patient’s mental status deteriorated to stupor. His condition continued to decline due to ongoing infection, ultimately leading to disseminated intravascular coagulation, acute kidney injury, and septic shock. Given this worsening general condition, further surgical intervention was considered unfeasible. Unfortunately, the patient died on POD 33.
Discussion
Recent evidence suggests that infections, particularly sepsis, may act as acute triggers for stroke, significantly increasing its risk within a short period [
5]. Potential mechanisms linking sepsis to stroke include atrial fibrillation, hemodynamic instability, coagulopathy, systemic inflammatory response syndrome, and prolonged inflammation [
6]. Thus, effective risk management requires identifying patients at the highest risk for stroke following sepsis and bloodstream infections. In the present case, the patient exhibited altered mental status postoperatively, and cerebral embolism was confirmed by brain CT. Among the most common high-risk cardiac conditions that can lead to embolic ischemic stroke is infective endocarditis [
7]. To investigate the presence of vegetation on the mitral valve and to determine if a thrombus was associated with atrial fibrillation, transthoracic echocardiography and coronary angiography were conducted. These tests revealed only stress-induced cardiomyopathy. Considering the patient’s condition, it is regrettable that transesophageal echocardiography was not performed to reassess for vegetation, which was not detected on transthoracic echocardiography. Additionally, given the patient’s overall state and vital signs, utilizing brain oximetry during anesthetic management may have provided valuable insights.
The use of low-dose aspirin is a common practice for the primary and secondary prevention of stroke [
8]. The present patient developed intraventricular hemorrhage in the right lateral ventricle and intracerebral hemorrhage in the right occipital lobe on POD 8, following the initiation of aspirin therapy. Although the need for antiplatelet therapy due to cerebral embolic infarction could have played a role, the patient exhibited persistently low platelet counts of 80,000–90,000/µL; instead, prolonged coagulopathy secondary to sepsis may have been the primary cause. Furthermore, hemorrhagic transformation of ischemic brain infarcts can result in intracerebral hemorrhage, especially in patients receiving anticoagulant therapy.
Sepsis requires the presence of infection, which may not be immediately evident in the perioperative setting. Patients with conditions such as perforated viscera or undrained abscesses require prompt surgical intervention to prevent exacerbation of the infection and severe systemic reactions [
9]. In the present case, MRI revealed an epidural or subdural abscess extending from the cervical to the lumbar level, necessitating immediate surgical intervention given the patient’s unstable vital signs. In cases of suspected sepsis, early recognition and focused management are critical. This includes the timely administration of antibiotics, intravenous fluids, and vasopressors (if hypotension is evident) to ensure adequate organ perfusion. Supportive measures such as monitoring urine output, providing high-flow oxygen, and tracking lactate levels are also important to prevent organ failure. In this case, empiric antibiotics were initiated preoperatively upon confirmation of infection, and vasopressors and intravenous fluids were administered intraoperatively, along with an increase in fraction of inspired oxygen. However, the patient’s HR remained persistently elevated at 110–140 beats per minute. Given the continued instability of his vital signs postoperatively, intraoperative administration of the class III antiarrhythmic medication amiodarone may have been beneficial. Amiodarone is particularly useful for hemodynamically unstable patients, as it effectively controls ventricular rate and promotes successful conversion to and maintenance of sinus rhythm in those with atrial fibrillation [
10].
Overall, the patient’s condition deteriorated due to ongoing infection and complications such as hemorrhagic events. This case underscores the importance of vigilant perioperative management, the early identification of patients at high risk, and the use of tailored therapeutic strategies. Comprehensive postoperative monitoring is essential to mitigate potential complications following surgery in patients with sepsis.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download