Abstract
Sexually transmitted infections (STIs) continue to pose significant public health
challenges in Korea, with syphilis, gonorrhea, chlamydia, Mycoplasma
genitalium, and herpes simplex virus (HSV) being the most
prevalent. This review provides an updated overview of the epidemiology,
diagnosis, and treatment of these significant STIs in Korea, highlighting recent
trends and concerns. Syphilis incidence rates have fluctuated due to changes in
surveillance systems. Starting in 2024, syphilis will be reclassified as a
nationally notifiable infectious disease (category 2). Gonorrhea remains a
concern due to increasing antibiotic resistance, including the emergence of
extensively drug-resistant Neisseria gonorrhoeae strains,
underscoring the need for vigilant antimicrobial stewardship. Chlamydia
continues to be the most commonly reported STI, although its incidence has
declined during the COVID-19 pandemic. M. genitalium has gained
attention as a significant STI with rising antibiotic resistance issues,
necessitating updated treatment guidelines and consideration of resistance
testing. HSV-2 remains a common cause of genital herpes, with steady incidence
rates reported. Updated diagnostic methods, including nucleic acid amplification
tests, and revised treatment guidelines are presented to effectively address
these infections. The impact of the COVID-19 pandemic on other STIs within Korea
remains unclear, necessitating further research. Changes in treatment
guidelines, such as the recommendation of doxycycline as first-line therapy for
chlamydia, reflect evolving evidence and resistance patterns. The importance of
updated diagnostic tools, including resistance testing for M.
genitalium, is emphasized to improve treatment outcomes. Continued
efforts in education, prevention, and research are essential to manage and
mitigate the impact of STIs on public health in Korea.
Go to : 
Sexually transmitted infections (STIs) are commonly encountered in outpatient
clinics. The major STIs primarily addressed in both domestic and international
treatment guidelines include syphilis, gonorrhea, chlamydia, and herpes simplex
virus (HSV) infection [1–4]. Recently, the incidence and significance
of Mycoplasma genitalium have been on the rise [5]. The COVID-19 pandemic has significantly
affected access to healthcare services, thereby altering the epidemiology of
major STIs. It has notably impacted access to HIV services, influencing
transmission rates [6,7]. Additionally, there has been a decline
in the incidence of new HIV cases in Korea after COVID-19 [8]. Some studies suggest that this reflects a genuine
reduction in incidence rates [8]. The
impact on other STIs within the country remains unclear.
This review focuses on syphilis, gonorrhea, chlamydia, M.
genitalium, and HSV. While genital warts or condyloma acuminata are
also prevalent STIs [9], they will be
addressed separately at a later date.
Go to : 
As this study is a literature review, it did not require institutional review board
approval or individual consent.
Go to : 
Syphilis is a disease that has been known for centuries, with documented cases
appearing in European and various other countries' records from the 11th to
15th centuries [10]. Historical documents
from the Joseon Dynasty in Korea also contain references to syphilis.
In Korea, syphilis is classified under the Infectious Disease Control and Prevention
Act as both a Category 4 communicable disease (Article 2, Item 5) and a STI (Item
10). It is monitored alongside six other STIs—gonorrhea, chlamydia infection,
chancroid, genital herpes, condyloma acuminatum, and human papillomavirus
infection—through designated sentinel surveillance institutions. However,
starting in 2024, it has been reclassified as a nationally notifiable infectious
disease (category 2 communicable disease), and data on its occurrence will be
collected once again [11]. The surveillance
system for syphilis has seen several changes over the years. Initially introduced in
2001, the sentinel surveillance system managed syphilis until 2010. It then
transitioned to full-scale surveillance following a reorganization of the legal
classification system for infectious diseases in 2010, only to revert to sentinel
surveillance in 2020.
The prevalence of syphilis in Korea from 2017 to 2020 is detailed below. In 2019,
comprehensive surveillance identified 1,750 syphilis cases, with 1,276 cases
(72.9%) occurring in males and 474 cases (27.1%) in females. The age group of
20–40 years had the highest incidence, with 1,281 cases (73.2%), while
those aged 60 and older accounted for 192 cases (11.0%). Regarding the stage of
the disease, there were 1,176 cases of primary syphilis, 554 cases of secondary
syphilis, and 23 cases of congenital syphilis. After shifting to sentinel
surveillance in 2020, a total of 330 cases were reported, comprising 228 cases
(69.1%) in males and 102 cases (30.9%) in females. The 20–40 age group
represented 251 cases (76.1%), and the 60 and older age group had 30 cases
(9.1%). The breakdown by stage included 191 cases of primary syphilis, 136 cases
of secondary syphilis, and 3 cases of congenital syphilis. These data were
sourced from the Weekly Health and Disease Report of the Korea Disease Control
and Prevention Agency. Since the transition to sentinel surveillance in 2021,
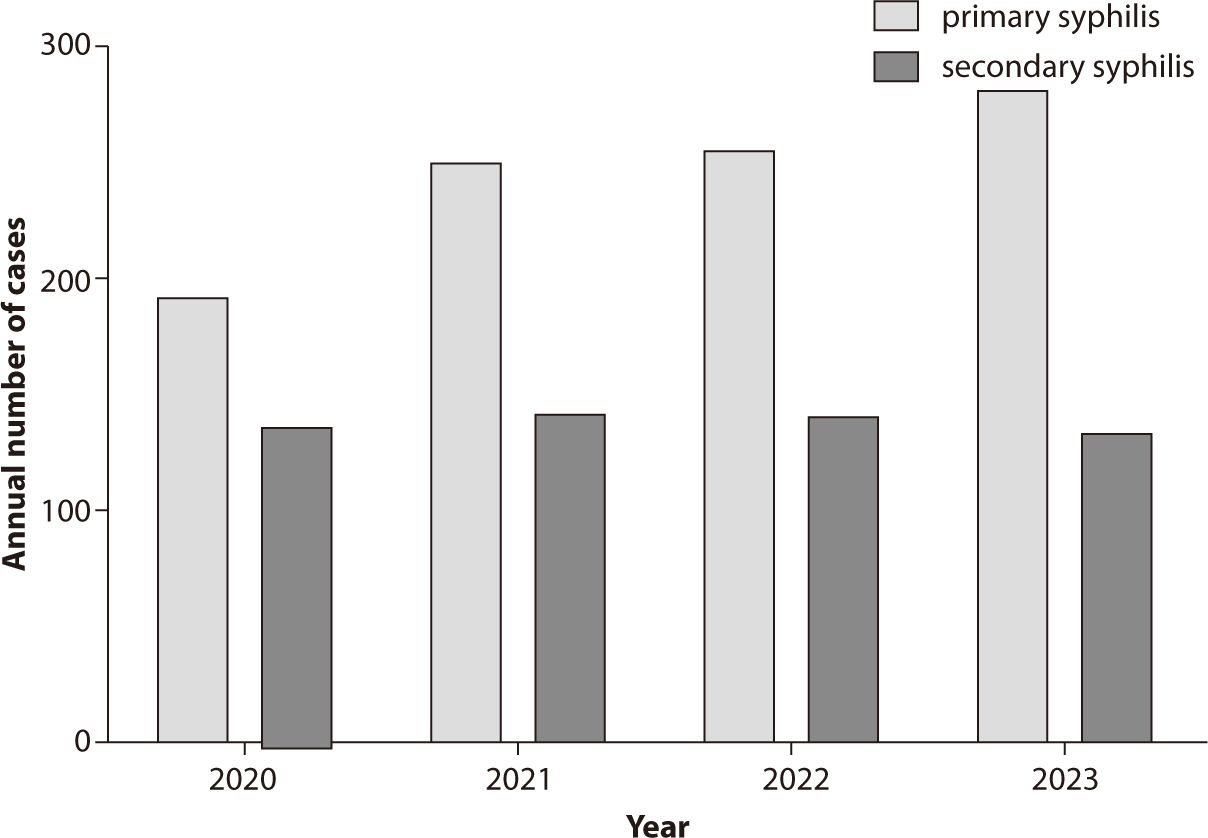
there has been a noticeable change in the number of reported cases. From 2020 to
2023, the reported cases of primary syphilis were 191, 248, 254, and 280,
respectively, while secondary syphilis cases were 136, 140, 138, and 131 for the
same years (Fig. 1). Starting in 2024, with
the resumption of full-scale surveillance, it will be possible to compare these
figures with previous data. Fig. 1 also
presents a detailed breakdown of the number of cases for each stage of
syphilis.
Syphilis is primarily transmitted through sexual contact or from an infected
mother to her child during pregnancy. This disease is caused by the spirochete
Treponema pallidum. Although less common, transmission can
also occur through blood transfusions or organ transplants. Additionally, there
is a minimal risk of transmission through needlestick injuries in certain stages
of the disease [12].
The clinical presentation of syphilis can be broadly divided into symptomatic
periods and asymptomatic latent syphilis periods. The clinical course of
syphilis is categorized into symptomatic phases and periods of asymptomatic
latent syphilis. The symptomatic stages include primary, secondary, and tertiary
syphilis, with periods of latent syphilis occurring between these stages.
Primary syphilis, the initial stage of infection, typically presents as a single
painless ulcer, known as a chancre, at the site of T. pallidum
entry. This sore usually appears within 9 to 90 days after exposure and may be
accompanied by localized swelling of lymph nodes. While the ulcer is generally
painless, it can occasionally be painful. The ulcer characteristic of primary
syphilis typically heals spontaneously.
Secondary syphilis typically develops 4–10 weeks after the appearance of
the primary lesion and can affect the entire body. The symptoms of secondary
syphilis are diverse, with a characteristic rash being a common feature. This
rash, frequently involving the palms and soles, is observed in 48%–70% of
patients with secondary syphilis. Other manifestations may include abdominal
pain, hepatitis, pulmonary nodules, and alopecia. Additionally, condyloma lata,
presenting as wart-like lesions, may also occur. Although many patients receive
treatment during the secondary stage, the condition might resolve on its own
without antibiotics; however, if left untreated, it can progress to latent
syphilis. During this stage, the bacteria may infiltrate the central nervous
system, potentially leading to meningitis. Approximately 70% of cases of
untreated or latent syphilis remain symptom-free, but some may advance to
tertiary syphilis. Tertiary syphilis can involve cardiovascular syphilis,
neurosyphilis, and gummatous syphilis. Neurosyphilis is divided into two primary
forms: meningeal neurosyphilis, which can present with cranial nerve
dysfunction, meningitis, stroke, and changes in hearing or vision, and tabes
dorsalis, characterized by demyelination of the dorsal columns and associated
with ataxia, diminished reflexes, impaired proprioception, neuropathic pain, and
paralysis. Typically, neurosyphilis develops 10–30 years after the
initial infection [13].
Diagnosing syphilis is complex. The most definitive method involves direct
evidence of the syphilis bacterium. However, because Treponema
is difficult to culture, a culture-based diagnosis is not feasible. Although
dark-field microscopy can visualize Treponema from lesion
samples, this method is rarely used today. In cases of primary syphilis, samples
from chancres can be tested using PCR or other nucleic acid amplification tests
(NAATs) to detect Treponema. Additionally, multiplex PCR tests,
which diagnose multiple STIs simultaneously, can also detect syphilis from urine
samples.
However, in cases of secondary, tertiary, and latent syphilis, it is not feasible
to obtain samples that confirm the presence of the pathogen. Therefore, the
diagnosis relies on a combination of tests. For initial screening,
non-treponemal tests such as the Venereal Disease Research Laboratories test or
the rapid plasma reagin test are employed. If these tests yield positive
results, confirmation is sought through treponemal tests, including the
T. pallidum latex agglutination, T.
pallidum hemagglutination assay, or the fluorescent treponemal
antibody absorption immunoglobulin test.
Both the Centers for Disease Control and Prevention 2021 treatment guidelines and
Korea's 2023 treatment guidelines recommend the same treatment [2]. Penicillin injections are the first-line
treatment for syphilis at all stages [14]. Benzathine penicillin G is the drug of choice for all cases except
neurosyphilis. Thanks to its depot effect, it can be administered once a week.
Benzathine penicillin G must be given intramuscularly, not intravenously. For
primary, secondary, and early latent syphilis, a single dose of 2.4 million
units of benzathine penicillin G is administered. For late latent syphilis, 2.4
million units are administered weekly for three weeks. Alternatively,
doxycycline 100 mg taken twice daily for 14–28 days can be used, but it
is contraindicated during pregnancy, where penicillin is the only option.
A characteristic reaction known as the Jarisch-Herxheimer reaction may occur
following the treatment of spirochetal infections, including syphilis [15]. This inflammatory response is
triggered by the release of cytokines and typically manifests within 24 hours of
treatment, presenting symptoms such as myalgia and fever.
Go to : 
Gonorrhea, similar to syphilis, is one of the oldest known STIs. The systematic
recording and management of gonorrhea in Korea commenced during the Japanese
colonial period and persisted through the American military government period
following liberation. Since that time, reports on domestic prevalence and antibiotic
susceptibility have been published [16].
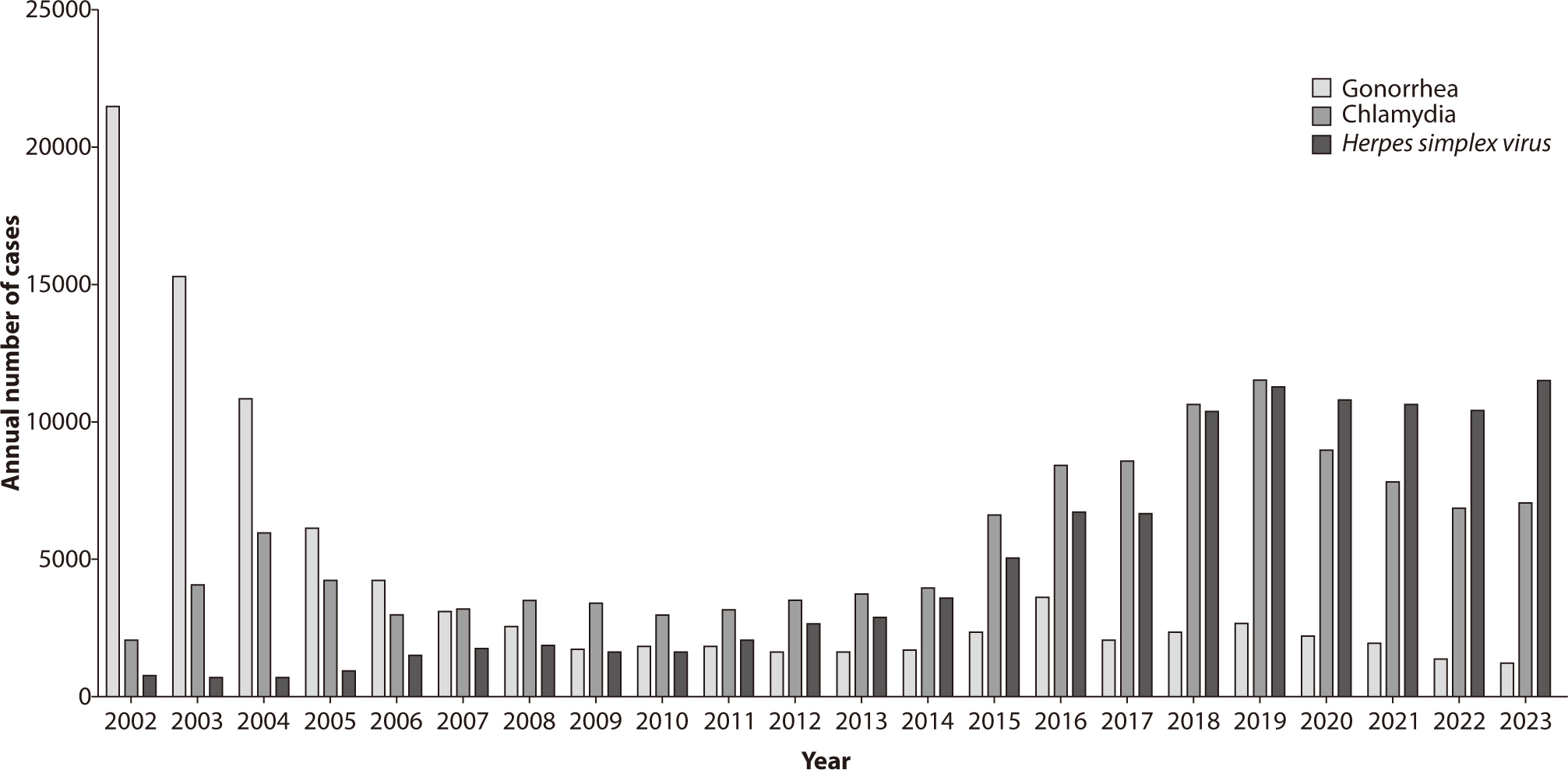
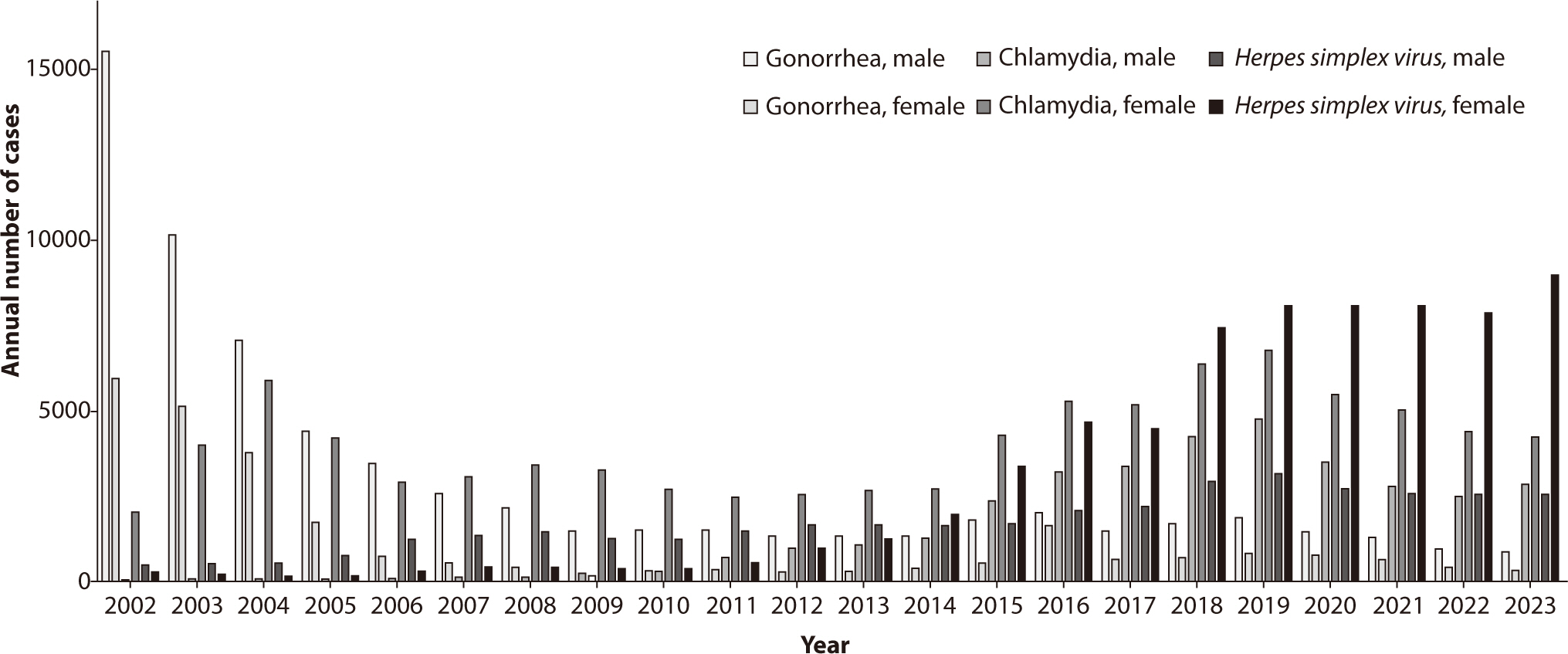
The annual incidence of gonorrhea in Korea is shown in Figs. 2, 3. In 2023,
sentinel surveillance reported 1,204 cases of gonorrhea, indicating a decreasing
trend in its incidence. A particularly concerning issue is the antibiotic
resistance of Neisseria gonorrhoeae, the bacterium responsible
for gonorrhea. There has been a significant global increase in resistance to
fluoroquinolones, including ciprofloxacin, and a rising trend in resistance to
azithromycin [17]. Additionally, strains
resistant to ceftriaxone, considered a last-resort treatment for gonorrhea, have
recently been identified, particularly in countries such as the United Kingdom
and Japan. Extensively drug-resistant N. gonorrhoeae has also
emerged, leading to treatment failures with the combined therapy of ceftriaxone
and azithromycin [18–20]. In Korea, the antibiotic resistance
rates of N. gonorrhoeae have shown concerning trends:
resistance to ciprofloxacin increased from 26% in 2000 to 83% in 2006 among
strains collected during that period. During the same timeframe, no resistance
to ceftriaxone or cefixime was reported. Resistance to tetracycline was noted to
be between 93%–100% [21]. From
data collected between 2011 and 2013, 3% of the strains were resistant to
ceftriaxone, 9% to cefixime, 38% showed intermediate resistance or resistance to
azithromycin, and 97% were resistant to ciprofloxacin [22]. Antibiotic resistance testing on N.
gonorrhoeae conducted by the Korea Centers for Disease Control and
Prevention from 2013 to 2015 indicated that almost no strains were susceptible
to ciprofloxacin and tetracycline. While susceptibility to ceftriaxone and
cefixime was still maintained, resistance to cefixime showed an increasing
trend. Furthermore, there was an upward trend in the number of strains
exhibiting simultaneous resistance to multiple antibiotics from 2013 to
2015.
Gonorrhea can result in infections in the urogenital tract, oropharynx, and
rectum. It can also cause bacteremia, which may manifest with skin lesions or
arthritis. In women, between 86.4% and 92.6% of urogenital infections caused by
gonorrhea are asymptomatic [23]. Although
less precisely determined, a population-based multicenter study with 11,408
participants found that 55.7% to 86.8% of urogenital infections in men were
asymptomatic [13,23].
The most common symptom of a gonorrhea infection is gonococcal urethritis, which
typically manifests in men as urethral discharge and dysuria following an
incubation period of 2 to 8 days. In women who exhibit symptoms, the infection
may lead to vaginal discharge, itching, intermenstrual bleeding, or heavy
menstrual periods. Pelvic inflammatory disease (PID) associated with gonorrhea
can cause symptoms such as abdominal pain or pain during intercourse. Gonococcal
pharyngitis may present with a sore throat, pharyngeal exudates, or swollen
cervical lymph nodes, whereas gonococcal proctitis can lead to anorectal pain, a
sensation of incomplete bowel evacuation, bleeding, or mucoid discharge. In
Korea, cases of disseminated gonococcal infection have been reported, presenting
as bacteremia and liver abscesses [24].
The typical presentation of disseminated gonococcal infection includes purulent
arthritis, polyarthralgia, tenosynovitis, and skin rashes [25].
The diagnosis of gonorrhea involves identifying N. gonorrhoeae
from the infected site. Traditionally, bacterial culture was the primary method
used; however, NAATs have now become widely employed. Nevertheless, to determine
antibiotic resistance, obtaining the pathogen through culture is necessary.
Therefore, it is recommended to perform bacterial culture alongside NAATs when
diagnosing gonorrhea. In men, first-void urine is commonly tested, and urethral
swab tests are also an option. In women, vaginal swab tests are primarily used,
although cervical swabs can also be taken. Recently, the use of self-collected
samples has been introduced. The sensitivity of culture for detecting gonorrhea
ranges from 50% to 85%, with lower sensitivity observed in non-genital sites and
among asymptomatic patients [26].
The treatment guidelines for gonorrhea are regularly updated. The most recent
guidelines from the U.S. Centers for Disease Control and Prevention (CDC),
issued in 2021, recommend a single intramuscular dose of 500 mg of ceftriaxone
for uncomplicated gonorrhea [2].
Additionally, if a chlamydial infection cannot be ruled out, administering
doxycycline (100 mg) twice daily for 7 days is advised. There are notable
differences between the U.S. and European guidelines, especially concerning the
use of dual therapy [4]. While previous
U.S. guidelines favored dual therapy, the current recommendations support
monotherapy. The 2023 domestic guidelines also endorse a single intramuscular or
intravenous dose of 500 mg ceftriaxone for uncomplicated urogenital, cervical,
and rectal gonococcal infections in adults. Alternative treatments include a
single intramuscular dose of 2 g of spectinomycin or a combination therapy
consisting of 240 mg of intramuscular gentamicin and 2 g of oral azithromycin.
For pharyngeal infections, however, both the U.S. and domestic guidelines
continue to recommend a single intramuscular dose of 500 mg of ceftriaxone.
Go to : 
Chlamydia is an infection caused by serovars D through K of Chlamydia
trachomatis. Previously, it was often categorized with other infections
such as non-gonococcal urethritis. However, it is now recognized as one of the most
prevalent STIs. In the United States, data from 2019 reported 1,808,703 new cases,
making chlamydia the most commonly reported STI [27]. In Korea, surveillance data indicated an increasing incidence of
chlamydia infections until 2019, with 11,518 cases reported that year. Following the
COVID-19 pandemic in 2020, however, the number of reported cases declined to 7,064
in 2023 (Figs. 2, 3). This decrease may be attributed to factors such as reduced testing
and healthcare access during the pandemic, changes in sexual behavior, or the
reprioritization of healthcare resources. These factors have been noted in various
international studies, although it was challenging to confirm with domestic data.
Nonetheless, it is believed that the situation in the country would not have been
significantly different.
Chlamydia can lead to urogenital, oropharyngeal, rectal, and ocular infections in
both men and women. The majority of these cases are asymptomatic, with over 70%
of urogenital infections in women and more than 80% in men presenting no
symptoms. Similarly, around 90% of rectal and pharyngeal infections remain
asymptomatic [28]. When symptoms do
occur, chlamydia infections typically manifest as urethritis, characterized by
dysuria and urethral discharge, cervicitis, PID, and proctitis, which includes
pain, discharge, and bleeding. In men, the infection may also lead to
epididymitis. Additionally, chlamydia can cause Fitz-Hugh-Curtis syndrome, a
specific form of PID.
In neonates born vaginally to mothers with chlamydia, there is a risk of
developing conjunctivitis or pneumonia. Additionally, a rare manifestation of
chlamydia infection, known as lymphogranuloma venereum (LGV), may also occur.
LGV progresses through three stages: initially, a small, painless, temporary
ulcer appears at the infection site. The second stage, which develops 2 to 6
weeks later, is marked by large, painful inguinal lymph nodes, with
approximately 30% of these nodes spontaneously rupturing. If left untreated, the
third stage involves chronic lymphadenitis, which leads to scarring, lymphedema,
and genital elephantiasis [29].
Chlamydia is diagnosed by detecting the pathogen with NAATs from the suspected
site of infection. The process for collecting samples is similar to that used
for gonorrhea; however, since cultures are not conducted, NAATs are the sole
diagnostic method employed.
The treatment for chlamydia is doxycycline. According to the updated 2021
guidelines from the U.S. CDC, doxycycline is now recommended as the first-line
treatment [2], a decision supported by
recent studies [30–32]. Domestic guidelines also endorse
doxycycline, prescribing 100 mg twice daily for 7 days. Alternative treatments
include azithromycin (1 g) or levofloxacin (500 mg), both administered for 7
days. A study conducted in Japan revealed that the antibiotic resistance rates
for C. trachomatis were 2.0% for azithromycin and 2.4% for
clarithromycin [33].
Go to : 
M. genitalium was not widely recognized as an infection in the past,
but it has recently gained attention as a STI. In the 2015 guidelines from the U.S.
CDC, M. genitalium was described as an "emerging pathogen of
uncertain significance" [34]. However,
with the accumulation of more data, recent guidelines now recommend treatment for it
[2].
Since M. genitalium is not a notifiable infection in Korea,
nationwide incidence statistics are not compiled. A study conducted in Korea
between 2012 and 2015 involving 14,932 soldiers with urological symptoms found
that chlamydia was the most prevalent infection at 36.6%, followed by
Ureaplasma urealyticum at 24.0%, and M.
genitalium at 21.5%. N. gonorrhoeae accounted for
19.0% of the cases [35]. Another study,
carried out from 2018 to 2020 on outpatients, identified M.
genitalium in 3.27% of the cases, with the highest prevalence
observed in the 20–29 age group [36].
The urogenital tract is the primary site of M. genitalium
infection, although it can also occasionally cause proctitis. In cases of
recurrent and persistent urethritis, M. genitalium is detected
in approximately 30%–40% of instances. Although M.
genitalium often remains asymptomatic, it is present in
30%–40% of men with persistent recurrent urethritis [37]. Symptoms during these episodes
typically include dysuria and urethral discomfort. In women, M.
genitalium is associated with cervicitis, PID, and infertility. The
main symptoms in women are those related to cervicitis, such as post-coital
bleeding, intermenstrual bleeding, and lower abdominal pain [38].
M. genitalium often fails to respond to treatment, primarily due
to resistance to the medications used. Therefore, recent major guidelines
recommend assessing resistance before selecting a treatment regimen for
M. genitalium [2].
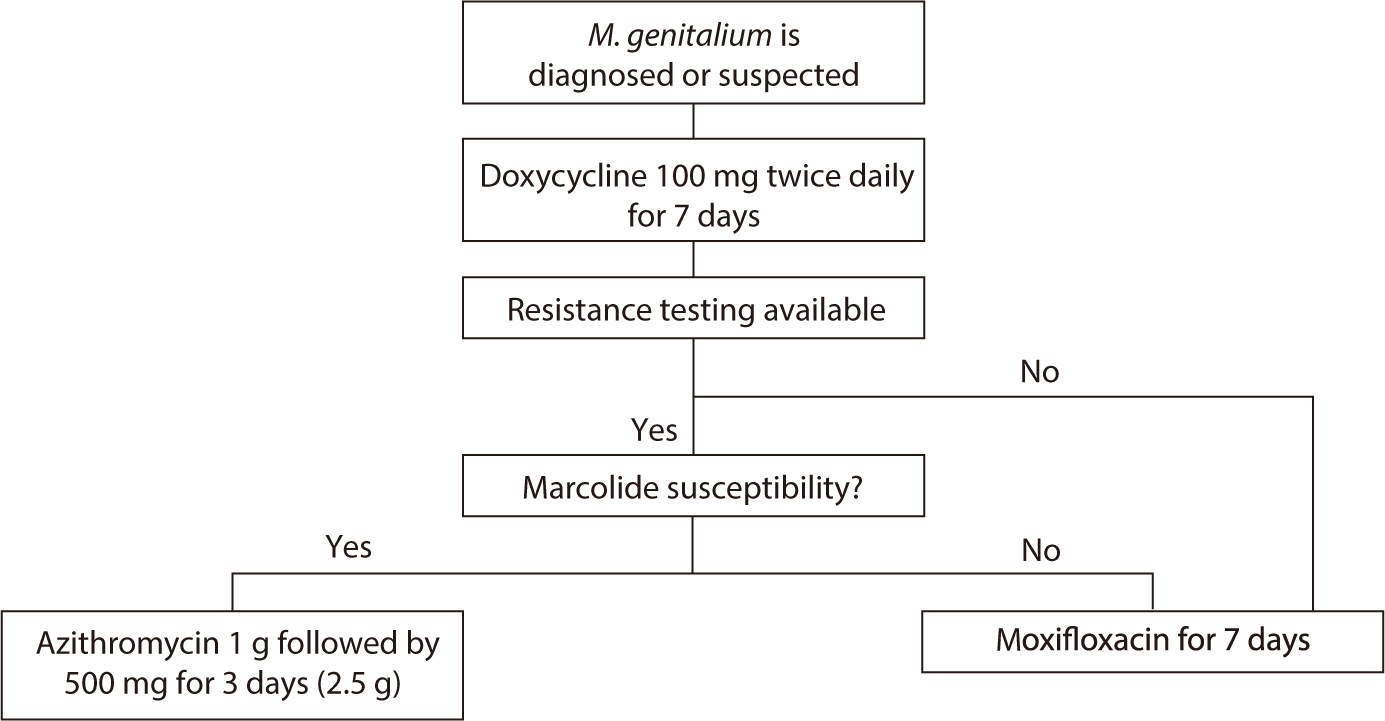
However, since macrolide resistance testing tools are not yet available
domestically, these recommendations cannot be applied in the local context
(Fig. 4).
If resistance testing is possible, administer doxycycline (100 mg) twice daily
for 7 days initially. If the organism is susceptible to macrolides, azithromycin
can be administered as a single 1 g oral dose followed by three additional 500
mg doses (totaling 2.5 g). In the presence of macrolide resistance, moxifloxacin
(400 mg) taken orally for 7 days is recommended. If resistance testing is not
available, the recommended approach is to administer doxycycline for one week
followed by moxifloxacin for another week. The 2023 domestic guidelines suggest
starting treatment with azithromycin (500 mg orally), followed by 250 mg daily
for 4 days (total 1.5 g). In cases of treatment failure or recurrence, a 7-day
course of doxycycline or minocycline is advised, followed by further treatment
decisions based on macrolide susceptibility. If testing is not feasible or if
macrolide susceptibility is confirmed, the recommended regimen is 1 g of
azithromycin followed by 500 mg daily for 3 days (total 2.5 g), with a follow-up
at 21 days to confirm cure. If macrolide resistance is detected, a 7-day course
of moxifloxacin 400 mg is recommended.
Go to : 
HSV is categorized into type 1 (HSV-1) and type 2 (HSV-2), both of which can
cause genital herpes [39]. However, HSV-2
is primarily associated with anogenital herpes, whereas HSV-1 typically leads to
cold sores around the lips. In Korea, the incidence of HSV-2 is monitored
through a sample surveillance system. Data show that there were 6,657 cases in
2017, 10,347 cases in 2018, 11,229 cases in 2019, 10,759 cases in 2020, 10,637
cases in 2021, 10,403 cases in 2022, and 11,449 cases in 2023. Additionally, the
number of cases reported per institution has been steadily increasing (Figs. 2, 3).
Most individuals infected with HSV remain asymptomatic, and the majority are
unaware that they are carriers. During the initial infection, which follows an
incubation period of approximately 4–7 days post-contact, patients
develop multiple painful, bilateral, erythematous lesions. These lesions
progress through stages including papules, vesicles, and ulcers, and typically
last between 16.5 to 19.7 days [13,40]. Additionally, 39%–68% of
patients report experiencing headaches, fever, and lymphadenopathy during this
initial phase [13]. Following the initial
outbreak, the virus enters a latent state where it remains asymptomatic.
Symptomatic recurrences occur in about 40-50% of cases and are often preceded by
prodromal symptoms localized to the affected area. These symptoms, which include
itching or burning sensations, help patients recognize the onset of a new
outbreak. Recurrences tend to be milder than the initial infection and usually
resolve within 5–10 days.
Testing for HSV can be performed on samples from vesicular lesions using NAATs,
such as PCR, which have very high sensitivity. A wet swab is the preferred
method, but a dry swab can also be used. While viral culture is an option, it is
less sensitive and more time-consuming than NAAT, and thus, it is not
recommended as the sole testing method. Viral culture may be utilized in
instances where the infection does not respond to treatment or antiviral
resistance is suspected. Serological testing is valuable for identifying
infections, especially latent infections in the absence of skin lesions, and is
frequently employed in demographic studies.
Treatment for HSV includes oral or injectable antiviral medications. The primary
antiviral drugs used are acyclovir, valacyclovir, and famciclovir. Although
these medications do not cure HSV, they help reduce symptoms, shorten the
duration of the disease, and decrease the recurrence rate. Despite the
availability of various antiviral drugs, most share similar mechanisms of action
and efficacy. For suspected initial infections, a 10-day course of antiviral
treatment is recommended, which may be extended by an additional week if lesions
persist. For recurrent lesions, a shorter course of treatment may be attempted.
Topical antiviral treatment alone is not recommended due to its insufficient
effectiveness.
Go to : 
This review highlights the evolving landscape of STIs in Korea. The COVID-19 pandemic
has significantly impacted the epidemiology of STIs, necessitating adaptations in
healthcare delivery and public health strategies. The emergence of
antibiotic-resistant strains, particularly in gonorrhea and M.
genitalium, underscores the importance of antimicrobial stewardship and
the development of new therapeutic options. Continuing efforts in education,
prevention, and research are essential to manage and mitigate the impact of STIs on
public health.
Go to : 
References
1. U.S. Department of Health and Human Services. Sexually transmitted infections national strategic plan for the United
States: 2021–2025. Washington: U.S. Department of Health and Human Services;2020.
2. Workowski KA. Sexually transmitted infections treatment guidelines,
2021. MMWR Recomm Rep. 2021; 70(4):1–187. DOI: 10.15585/mmwr.rr7004a1. PMID: 34292926. PMCID: PMC8344968.
3. World Health Organization. Guidelines for the management of symptomatic sexually transmitted
infections. Geneva: World Health Organization;2021.
4. Gamoudi D, Flew S, Cusini M, Benardon S, Poder A, Radcliffe K. 2018 European guideline on the organization of a consultation for
sexually transmitted infections. J Eur Acad Dermatol Venereol. 2019; 33(8):1452–1458. DOI: 10.1111/jdv.15577. PMID: 30968975.

5. Wood GE, Bradshaw CS, Manhart LE. Update in epidemiology and management of Mycoplasma
genitalium infections. Infect Dis Clin North Am. 2023; 37(2):311–333. DOI: 10.1016/j.idc.2023.02.009. PMID: 37105645.

6. Lee J, Kim Y, Choi JY. Impact of the COVID-19 pandemic on HIV services in Korea: results
from a cross-sectional online survey. Infect Chemother. 2021; 53(4):741–752. DOI: 10.3947/ic.2021.0112. PMID: 34979605. PMCID: PMC8731254.

7. Seong H, Choi Y, Ahn KH, Choi JY, Kim SW, Kim SI, et al. Assessment of disease burden and immunization rates for
vaccine-preventable diseases in people living with HIV: the Korea HIV/AIDS
cohort study. Infect Chemother. 2023; 55(4):441–450. DOI: 10.3947/ic.2023.0045. PMID: 37674339. PMCID: PMC10771952.

8. Lee M, Park WB, Kim ES, Kim Y, Park SW, Lee E, et al. Possibility of decreasing incidence of human immunodeficiency
virus infection in Korea. Infect Chemother. 2023; 55(4):451–459. DOI: 10.3947/ic.2023.0056. PMID: 37674340. PMCID: PMC10771950.

9. Park JK, Shin YS. Risk factors for urethral condyloma among heterosexual young male
patients with condyloma acuminatum of penile skin. Infect Chemother. 2016; 48(3):216–218. DOI: 10.3947/ic.2016.48.3.216. PMID: 27659432. PMCID: PMC5048003.

10. Rothschild BM. History of syphilis. Clin Infect Dis. 2005; 40(10):1454–1463. DOI: 10.1086/429626. PMID: 15844068.

11. Wang S, Kim S, Cho S, Kim HS, Min S. Introduction to the transition of mandatory surveillance system
in the syphilis monitoring. Public Health Wkly Rep. 2023; 16(47):1620–1630. DOI: 10.56786/PHWR.2023.16.47.3.
12. Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020; 382(9):845–854. DOI: 10.1056/NEJMra1901593. PMID: 32101666.

13. Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: a
review. JAMA. 2022; 327(2):161–172. DOI: 10.1001/jama.2021.23487. PMID: 35015033.

14. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014; 312(18):1905–1917. DOI: 10.1001/jama.2014.13259. PMID: 25387188. PMCID: PMC6690208.
15. Butler T. The Jarisch–Herxheimer reaction after antibiotic treatment
of spirochetal infections: a review of recent cases and our understanding of
pathogenesis. Am J Trop Med Hyg. 2017; 96(1):46–52. DOI: 10.4269/ajtmh.16-0434. PMID: 28077740. PMCID: PMC5239707.

16. Choi JK, Lee SJ, Yoo JH. History of syphilis and gonorrhea in Korea. Infect Chemother. 2019; 51(2):210–216. DOI: 10.3947/ic.2019.51.2.210. PMID: 31271002. PMCID: PMC6609744.

17. Unemo M, Lahra MM, Escher M, Eremin S, Cole MJ, Galarza P, et al. WHO global antimicrobial resistance surveillance for
Neisseria gonorrhoeae 2017–18: a retrospective
observational study. Lancet Microbe. 2021; 2(11):E627–E636. DOI: 10.1016/S2666-5247(21)00171-3. PMID: 35544082.
18. Kueakulpattana N, Wannigama DL, Luk-in S, Hongsing P, Hurst C, Badavath VN, et al. Multidrug-resistant Neisseria gonorrhoeae
infection in heterosexual men with reduced susceptibility to ceftriaxone,
first report in Thailand. Sci Rep. 2021; 11(1):21659. DOI: 10.1038/s41598-021-00675-y. PMID: 34737332. PMCID: PMC8569152.
19. Pleininger S, Indra A, Golparian D, Heger F, Schindler S, Jacobsson S, et al. Extensively drug-resistant (XDR) Neisseria
gonorrhoeae causing possible gonorrhoea treatment failure with
ceftriaxone plus azithromycin in Austria, April 2022. Eurosurveill. 2022; 27(24):2200455. DOI: 10.2807/1560-7917.ES.2022.27.24.2200455. PMID: 35713023. PMCID: PMC9205165.
20. Yun IJ, Park HJ, Chae J, Heo SJ, Kim YC, Kim B, et al. Nationwide analysis of antimicrobial prescription in Korean
hospitals between 2018 and 2021: the 2023 KONAS report. Infect Chemother. 2024; 56(2):256–265. DOI: 10.3947/ic.2024.0013. PMID: 38960739. PMCID: PMC11224044.

21. Lee H, Hong SG, Soe Y, Yong D, Jeong SH, Lee K, et al. Trends in antimicrobial resistance of Neisseria
gonorrhoeae isolated from Korean patients from 2000 to
2006. Sex Transm Dis. 2011; 38(11):1082–1086. DOI: 10.1097/OLQ.0b013e31822e60a4. PMID: 21992988.

22. Lee H, Unemo M, Kim HJ, Seo Y, Lee K, Chong Y. Emergence of decreased susceptibility and resistance to
extended-spectrum cephalosporins in Neisseria gonorrhoeae
in Korea. J Antimicrob Chemother. 2015; 70(9):2536–2542. DOI: 10.1093/jac/dkv146. PMID: 26084303. PMCID: PMC4539094.

23. Detels R, Green AM, Klausner JD, Katzenstein D, Gaydos C, Handsfield H, et al. The incidence and correlates of symptomatic and asymptomatic
Chlamydia trachomatis and Neisseria
gonorrhoeae infections in selected populations in five
countries. Sex Transm Dis. 2011; 38(6):503–509. DOI: 10.1097/OLQ.0b013e318206c288. PMID: 22256336. PMCID: PMC3408314.

24. Lee MH, Byun J, Jung M, Yang JJ, Park KH, Moon S, et al. Disseminated gonococcal infection presenting as bacteremia and
liver abscesses in a healthy adult. Infect Chemother. 2015; 47(1):60–63. DOI: 10.3947/ic.2015.47.1.60. PMID: 25844265. PMCID: PMC4384456.

25. Nettleton WD, Kent JB, Macomber K, Brandt MG, Jones K, Ridpath AD, et al. Notes from the field: ongoing cluster of highly related
disseminated gonococcal infections: Southwest Michigan, 2019. Morb Mortal Wkly Rep. 2020; 69(12):353–354. DOI: 10.15585/mmwr.mm6912a5. PMID: 32214085. PMCID: PMC7725508.

26. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of
Chlamydia trachomatis and Neisseria
gonorrhoeae: 2014. MMWR Recomm Rep. 2014; 63(RR02):1–19.
27. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2018. Atlanta: U.S. Department of Health and Human Services;2019.
28. Chan PA, Robinette A, Montgomery M, Almonte A, Cu-Uvin S, Lonks JR, et al. Extragenital infections caused by Chlamydia
trachomatis and Neisseria gonorrhoeae: a
review of the literature. Infect Dis Obstet Gynecol. 2016; 2016(1):5758387. DOI: 10.1155/2016/5758387. PMID: 27366021. PMCID: PMC4913006.
29. Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis,
and treatment. Clin Infect Dis. 2015; 61(suppl_8):S865–S873. DOI: 10.1093/cid/civ756. PMID: 26602624.
30. Geisler WM, Uniyal A, Lee JY, Lensing SY, Johnson S, Perry RCW, et al. Azithromycin versus doxycycline for urogenital chlamydia
trachomatis infection. N Engl J Med. 2015; 373(26):2512–2521. DOI: 10.1056/NEJMoa1502599. PMID: 26699167. PMCID: PMC4708266.

31. Dombrowski JC, Wierzbicki MR, Newman LM, Powell JA, Miller A, Dithmer D, et al. Doxycycline versus azithromycin for the treatment of rectal
chlamydia in men who have sex with men: a randomized controlled
trial. Clin Infect Dis. 2021; 73(5):824–831. DOI: 10.1093/cid/ciab153. PMID: 33606009. PMCID: PMC8571563.

32. Lau A, Kong FYS, Fairley CK, Templeton DJ, Amin J, Phillips S, et al. Azithromycin or doxycycline for asymptomatic rectal
Chlamydia trachomatis. N Engl J Med. 2021; 384(25):2418–2427. DOI: 10.1056/NEJMoa2031631. PMID: 34161706.

33. Suzuki S, Hoshi S, Sagara Y, Sekizawa A, Kinoshita K, Kitamura T. Antimicrobial resistance for Chlamydia
trachomatis genital infection during pregnancy in
Japan. Infect Chemother. 2022; 54(1):173–175. DOI: 10.3947/ic.2022.0030. PMID: 35384428. PMCID: PMC8987183.

34. Workowski KA. Centers for disease control and prevention sexually transmitted
diseases treatment guidelines. Clin Infect Dis. 2015; 61(suppl_8):S759–S762. DOI: 10.1093/cid/civ771. PMID: 26602614.
35. Kim HJ, Park JK, Park SC, Kim YG, Choi H, Ko JI, et al. The prevalence of causative organisms of community-acquired
urethritis in an age group at high risk for sexually transmitted infections
in Korean soldiers. BMJ Mil Health. 2017; 163(1):20–22. DOI: 10.1136/jramc-2015-000488. PMID: 26607860.

36. Oh EJ, Jang TS, Kim JK. Mycoplasma genitalium and Mycoplasma
hominis infection in South Korea during
2018-2020. Iran J Microbiol. 2021; 13(5):602–607. DOI: 10.18502/ijm.v13i5.7423. PMID: 34900157. PMCID: PMC8629812.
37. Seña AC, Lensing S, Rompalo A, Taylor SN, Martin DH, Lopez LM, et al. Chlamydia trachomatis, Mycoplasma genitalium,
and Trichomonas vaginalis infections in men with
nongonococcal urethritis: predictors and persistence after
therapy. J Infect Dis. 2012; 206(3):357–365. DOI: 10.1093/infdis/jis356. PMID: 22615318. PMCID: PMC3490700.
38. Soni S, Horner P, Rayment M, Pinto-Sander N, Naous N, Parkhouse A, et al. British Association for Sexual Health and HIV national guideline
for the management of infection with Mycoplasma genitalium
(2018). Int J STD AIDS. 2019; 30(10):938–950. DOI: 10.1177/0956462419825948. PMID: 31280688.

39. Gnann JW Jr, Whitley RJ. Genital herpes. N Engl J Med. 2016; 375(19):1906. DOI: 10.1056/NEJMc1611877.

40. Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations,
course, and complications. Ann Intern Med. 1983; 98(6):958–972. DOI: 10.7326/0003-4819-98-6-958. PMID: 6344712.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download