1. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge
and future perspectives consensus statement from the European Network for
the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016; 13(5):261–280. DOI:
10.1038/nrgastro.2016.51. PMID:
27095655.

2. European Association for the Study of the Liver. EASL-ILCA clinical practice guidelines on the management of
intrahepatic cholangiocarcinoma. J Hepatol. 2023; 79(1):181–208. DOI:
10.1016/j.jhep.2023.09.006. PMID:
37748953.
3. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and
management. Nat Rev Gastroenterol Hepatol. 2020; 17(9):557–588. DOI:
10.1038/s41575-020-0310-z. PMID:
32606456. PMCID:
PMC7447603.

4. Yang JQ, Wang XG, Wu B. Incidence trend and prognosis of intrahepatic cholangiocarcinoma:
a study based on the SEER database. Transl Cancer Res. 2023; 12(11):3007–3015. DOI:
10.21037/tcr-23-1278. PMID:
38130317. PMCID:
PMC10731349.

5. Seo N, Kim DY, Choi JY. Cross-sectional imaging of intrahepatic cholangiocarcinoma:
development, growth, spread, and prognosis. AJR Am J Roentgenol. 2017; 209(2):W64–W75. DOI:
10.2214/AJR.16.16923. PMID:
28570102.
6. Zhang Y, Uchida M, Abe T, Nishimura H, Hayabuchi N, Nakashima Y. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic
CT and dynamic MRI. J Comput Assist Tomogr. 1999; 23(5):670–677. DOI:
10.1097/00004728-199909000-00004. PMID:
10524843.

7. Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement
patterns on gadoxetic acid–enhanced MR images. Radiology. 2012; 264(3):751–760. DOI:
10.1148/radiol.12112308. PMID:
22798225.

8. Maetani Y, Itoh K, Watanabe C, Shibata T, Ametani F, Yamabe H, et al. MR imaging of intrahepatic cholangiocarcinoma with pathologic
correlation. AJR Am J Roentgenol. 2001; 176(6):1499–1507. DOI:
10.2214/ajr.176.6.1761499. PMID:
11373220.

9. Nam JG, Lee JM, Joo I, Ahn SJ, Park JY, Lee KB, et al. Intrahepatic mass-forming cholangiocarcinoma: relationship
between computed tomography characteristics and histological
subtypes. J Comput Assist Tomogr. 2018; 42(3):340–349. DOI:
10.1097/RCT.0000000000000695. PMID:
29189405.

10. Rhee H, Kim MJ, Park YN, An C. A proposal of imaging classification of intrahepatic mass-forming
cholangiocarcinoma into ductal and parenchymal types: clinicopathologic
significance. Eur Radiol. 2019; 29(6):3111–3121. DOI:
10.1007/s00330-018-5898-9. PMID:
30560357.

11. Bosman FT, Carneiro F, Hruban R, Theise N. WHO classification of tumours: digestive system tumours. 5th ed. Geneva: World Health Organization;2019.
13. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma
based on a new concept. World J Hepatol. 2010; 2(12):419–427. DOI:
10.4254/wjh.v2.i12.419. PMID:
21191517. PMCID:
PMC3010511.

14. Aishima S, Oda Y. Pathogenesis and classification of intrahepatic
cholangiocarcinoma: different characters of perihilar large duct type versus
peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015; 22(2):94–100. DOI:
10.1002/jhbp.154. PMID:
25181580.

15. Jeon Y, Kwon SM, Rhee H, Yoo JE, Chung T, Woo HG, et al. Molecular and radiopathologic spectrum between HCC and
intrahepatic cholangiocarcinoma. Hepatology. 2023; 77(1):92–108. DOI:
10.1002/hep.32397. PMID:
35124821.

16. Chung T, Rhee H, Nahm JH, Jeon Y, Yoo JE, Kim YJ, et al. Clinicopathological characteristics of intrahepatic
cholangiocarcinoma according to gross morphologic type: cholangiolocellular
differentiation traits and inflammation- and
proliferation-phenotypes. HPB. 2020; 22(6):864–873. DOI:
10.1016/j.hpb.2019.10.009. PMID:
31735647.

17. Nakanuma Y, Sasaki M, Sato Y, Ren X, Ikeda H, Harada K. Multistep carcinogenesis of perihilar cholangiocarcinoma arising
in the intrahepatic large bile ducts. World J Hepatol. 2009; 1(1):35–42. DOI:
10.4254/wjh.v1.i1.35. PMID:
21160963. PMCID:
PMC2999259.

18. Chung T, Park YN. Up-to-date pathologic classification and molecular
characteristics of intrahepatic cholangiocarcinoma. Front Med. 2022; 9:857140. DOI:
10.3389/fmed.2022.857140. PMID:
35433771. PMCID:
PMC9008308.
19. Li Z, Nguyen Canh H, Takahashi K, Le Thanh D, Nguyen Thi Q, Yang R, et al. Histopathological growth pattern and vessel co-option in
intrahepatic cholangiocarcinoma. Med Mol Morphol. 2024; 57(3):200–217. DOI:
10.1007/s00795-024-00392-1. PMID:
38960952. PMCID:
PMC11343874.

20. Sugita H, Nakanuma S, Gabata R, Tokoro T, Takei R, Okazaki M, et al. Clinicopathological features of cholangiolocarcinoma and impact
of tumor heterogeneity on prognosis: a single institution retrospective
study. Oncol Lett. 2024; 27(5):213. DOI:
10.3892/ol.2024.14346. PMID:
38572060. PMCID:
PMC10988194.
21. Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, et al. Distinct clinicopathologic and genetic features of 2 histologic
subtypes of intrahepatic cholangiocarcinoma. Am J Surg Pathol. 2016; 40(8):1021–1030. DOI:
10.1097/PAS.0000000000000670. PMID:
27259014.

22. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to
build a bridge from a population-based to a more
"personalized" approach to cancer staging. CA Cancer J Clin. 2017; 67(2):93–99. DOI:
10.3322/caac.21388. PMID:
28094848.

23. Kim Y, Moris DP, Zhang XF, Bagante F, Spolverato G, Schmidt C, et al. Evaluation of the 8th edition American Joint Commission on Cancer
(AJCC) staging system for patients with intrahepatic cholangiocarcinoma: a
surveillance, epidemiology, and end results (SEER) analysis. J Surg Oncol. 2017; 116(6):643–650. DOI:
10.1002/jso.24720. PMID:
28608424.

24. Spolverato G, Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Comparative performances of the 7th and the 8th editions of the
American Joint Committee on Cancer staging systems for intrahepatic
cholangiocarcinoma. J Surg Oncol. 2017; 115(6):696–703. DOI:
10.1002/jso.24569. PMID:
28194791.

25. Kang SH, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Prognostic comparison of the 7th and 8th editions of the American
Joint Committee on Cancer staging system for intrahepatic
cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2018; 25(4):240–248. DOI:
10.1002/jhbp.543. PMID:
29450978.

26. Kim YY, Yeom SK, Shin H, Choi SH, Rhee H, Park JH, et al. Clinical staging of mass‐forming intrahepatic
cholangiocarcinoma: computed tomography versus magnetic resonance
imaging. Hepatol Commun. 2021; 5(12):2009–2018. DOI:
10.1002/hep4.1774. PMID:
34559470. PMCID:
PMC8631089.

27. Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic
cholangiocarcinoma. Surgery. 2013; 153(6):811–818. DOI:
10.1016/j.surg.2012.12.005. PMID:
23499016. PMCID:
PMC3980567.

28. Ali SM, Clark CJ, Mounajjed T, Wu TT, Harmsen WS, Reid‐Lombardo KM, et al. Model to predict survival after surgical resection of
intrahepatic cholangiocarcinoma: the Mayo Clinic experience. HPB. 2015; 17(3):244–250. DOI:
10.1111/hpb.12333. PMID:
25410716. PMCID:
PMC4333786.

29. Hwang S, Lee YJ, Song GW, Park KM, Kim KH, Ahn CS, et al. Prognostic impact of tumor growth type on 7th AJCC staging system
for intrahepatic cholangiocarcinoma: a single-center experience of 659
cases. J Gastrointest Surg. 2015; 19(7):1291–1304. DOI:
10.1007/s11605-015-2803-6. PMID:
25820487.

30. Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, et al. Recurrence patterns and disease-free survival after resection of
intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic
models. J Am Coll Surg. 2016; 223(3):493–505e2. DOI:
10.1016/j.jamcollsurg.2016.05.019. PMID:
27296525. PMCID:
PMC5003652.
31. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic
cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014; 149(6):565–574. DOI:
10.1001/jamasurg.2013.5137. PMID:
24718873.

32. Tabrizian P, Jibara G, Hechtman JF, Franssen B, Labow DM, Schwartz ME, et al. Outcomes following resection of intrahepatic
cholangiocarcinoma. HPB. 2015; 17(4):344–351. DOI:
10.1111/hpb.12359. PMID:
25395176. PMCID:
PMC4368399.

33. Olthof SC, Othman A, Clasen S, Schraml C, Nikolaou K, Bongers M. Imaging of cholangiocarcinoma. Visc Med. 2016; 32(6):402–410. DOI:
10.1159/000453009. PMID:
28229074. PMCID:
PMC5290452.

34. Péporté ARJ, Sommer WH, Nikolaou K, Reiser MF, Zech CJ. Imaging features of intrahepatic cholangiocarcinoma in
Gd-EOB-DTPA-enhanced MRI. Eur J Radiol. 2013; 82(3):E101–E106. DOI:
10.1016/j.ejrad.2012.10.010. PMID:
23159401.
35. Marion-Audibert AM, Vullierme MP, Ronot M, Mabrut JY, Sauvanet A, Zins M, et al. Routine MRI with DWI sequences to detect liver metastases in
patients with potentially resectable pancreatic ductal carcinoma and normal
liver CT: a prospective multicenter study. AJR Am J Roentgenol. 2018; 211(5):W217–W225. DOI:
10.2214/AJR.18.19640. PMID:
30240298.
36. Shao C, Chen J, Chen J, Shi J, Huang L, Qiu Y. Histological classification of microvascular invasion to predict
prognosis in intrahepatic cholangiocarcinoma. Int J Clin Exp Pathol. 2017; 10(7):7674–7681.
37. Hu LS, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, et al. Impact of microvascular invasion on clinical outcomes after
curative-intent resection for intrahepatic
cholangiocarcinoma. J Surg Oncol. 2019; 119(1):21–29. DOI:
10.1002/jso.25305. PMID:
30466151.

38. Zhou Y, Wang X, Xu C, Zhou G, Liu X, Gao S, et al. Mass-forming intrahepatic cholangiocarcinoma: can
diffusion-weighted imaging predict microvascular invasion? J Magn Reson Imaging. 2019; 50(1):315–324. DOI:
10.1002/jmri.26566. PMID:
30444023.

39. Ma X, Liu L, Fang J, Rao S, Lv L, Zeng M, et al. MRI features predict microvascular invasion in intrahepatic
cholangiocarcinoma. Cancer Imaging. 2020; 20(1):40. DOI:
10.1186/s40644-020-00318-x. PMID:
32576283. PMCID:
PMC7310524.
40. Zhou Y, Zhou G, Zhang J, Xu C, Wang X, Xu P. Radiomics signature on dynamic contrast-enhanced MR images: a
potential imaging biomarker for prediction of microvascular invasion in
mass-forming intrahepatic cholangiocarcinoma. Eur Radiol. 2021; 31(9):6846–6855. DOI:
10.1007/s00330-021-07793-1. PMID:
33638019.

41. Kawarada Y, Yamagiwa K, Das BC. Analysis of the relationships between clinicopathologic factors
and survival time in intrahepatic cholangiocarcinoma. Am J Surg. 2002; 183(6):679–685. DOI:
10.1016/S0002-9610(02)00853-X. PMID:
12095601.

43. Yamamoto Y, Turkoglu MA, Aramaki T, Sugiura T, Okamura Y, Ito T, et al. Vascularity of intrahepatic cholangiocarcinoma on computed
tomography is predictive of lymph node metastasis. Ann Surg Oncol. 2016; 23:Suppl 4. 485–493. DOI:
10.1245/s10434-016-5382-1. PMID:
27393571.

44. Meng ZW, Lin XQ, Zhu JH, Han SH, Chen YL. A nomogram to predict lymph node metastasis before resection in
intrahepatic cholangiocarcinoma. J Surg Res. 2018; 226:56–63. DOI:
10.1016/j.jss.2018.01.024. PMID:
29661289.

45. Tsilimigras DI, Sahara K, Paredes AZ, Moro A, Mehta R, Moris D, et al. Predicting lymph node metastasis in intrahepatic
cholangiocarcinoma. J Gastrointest Surg. 2021; 25(5):1156–1163. DOI:
10.1007/s11605-020-04720-5. PMID:
32757124.
46. Rhee H, Lim HJ, Han K, Yeom SK, Choi SH, Park JH, et al. A preoperative scoring system to predict lymph node metastasis in
intrahepatic cholangiocarcinoma. Hepatol Int. 2023; 17(4):942–953. DOI:
10.1007/s12072-022-10477-7. PMID:
36689090.

47. Huang X, Yang J, Li J, Xiong Y. Comparison of magnetic resonance imaging and 18-fludeoxyglucose
positron emission tomography/computed tomography in the diagnostic accuracy
of staging in patients with cholangiocarcinoma: a
meta-analysis. Medicine. 2020; 99(35):e20932. DOI:
10.1097/MD.0000000000020932. PMID:
32871859. PMCID:
PMC7458197.
48. Park S, Lee Y, Kim H, Yu MH, Lee ES, Yoon JH, et al. Subtype classification of intrahepatic cholangiocarcinoma using
liver MR imaging features and its prognostic value. Liver Cancer. 2022; 11(3):233–246. DOI:
10.1159/000521747. PMID:
35949291. PMCID:
PMC9218635.
49. Park MS. Review of mass-forming intrahepatic
cholangiocarcinoma. Korean J Abdom Radiol. 2022; 6(1):1–11. DOI:
10.52668/kjar.2022.00150.

50. Zen Y. Intrahepatic cholangiocarcinoma: typical features, uncommon
variants, and controversial related entities. Hum Pathol. 2023; 132:197–207. DOI:
10.1016/j.humpath.2022.06.001. PMID:
35697170.

51. Giambelluca D, Cutaia G, Midiri M, Salvaggio G. The "pruned tree" appearance of primary sclerosing
cholangitis. Abdom Radiol. 2019; 44(8):2935–2936. DOI:
10.1007/s00261-019-02026-y. PMID:
31025068.

52. Giambelluca D, Leto C, D'Arpa F, Midiri M, Salvaggio G. Beaded bile ducts in primary sclerosing
cholangitis. Abdom Radiol. 2019; 44(3):1195–1196. DOI:
10.1007/s00261-018-1873-9. PMID:
30600377.

54. Ariizumi SI, Kotera Y, Takahashi Y, Katagiri S, Chen IP, Ota T, et al. Mass-forming intrahepatic cholangiocarcinoma with marked
enhancement on arterial-phase computed tomography reflects favorable
surgical outcomes. J Surg Oncol. 2011; 104(2):130–139. DOI:
10.1002/jso.21917. PMID:
21448898.

55. Nanashima A, Abo T, Murakami G, Matsumoto A, Tou K, Takeshita H, et al. Intrahepatic cholangiocarcinoma: relationship between tumor
imaging enhancement by measuring attenuation and clinicopathologic
characteristics. Abdom Imaging. 2013; 38(4):785–792. DOI:
10.1007/s00261-012-9974-3. PMID:
23232581.

56. Min JH, Kim YK, Choi SY, Kang TW, Lee SJ, Kim JM, et al. Intrahepatic mass-forming cholangiocarcinoma: arterial
enhancement patterns at MRI and prognosis. Radiology. 2019; 290(3):691–699. DOI:
10.1148/radiol.2018181485. PMID:
30620253.

57. Yugawa K, Itoh S, Yoshizumi T, Iseda N, Tomiyama T, Toshima T, et al. Prognostic impact of tumor microvessels in intrahepatic
cholangiocarcinoma: association with tumor-infiltrating
lymphocytes. Mod Pathol. 2021; 34(4):798–807. DOI:
10.1038/s41379-020-00702-9. PMID:
33077921.

58. Vigano L, Soldani C, Franceschini B, Cimino M, Lleo A, Donadon M, et al. Tumor-infiltrating lymphocytes and macrophages in intrahepatic
cholangiocellular carcinoma. Impact on prognosis after complete
surgery. J Gastrointest Surg. 2019; 23(11):2216–2224. DOI:
10.1007/s11605-019-04111-5. PMID:
30843133.

59. Rhee H, Park JH, Park YN. Update on pathologic and radiologic diagnosis of combined
hepatocellular-cholangiocarcinoma. J Liver Cancer. 2021; 21(1):12–24. DOI:
10.17998/jlc.21.1.12. PMID:
37384273. PMCID:
PMC10035725.

60. Kajiyama K, Maeda T, Takenaka K, Sugimachi K, Tsuneyoshi M. The significance of stromal desmoplasia in intrahepatic
cholangiocarcinoma: a special reference of 'scirrhous-type'
and 'nonscirrhous-type' growth. Am J Surg Pathol. 1999; 23(8):892–902. DOI:
10.1097/00000478-199908000-00006. PMID:
10435558.

61. Lacomis JM, Baron RL, Oliver JH 3rd, Nalesnik MA, Federle MP. Cholangiocarcinoma: delayed CT contrast enhancement
patterns. Radiology. 1997; 203(1):98–104. DOI:
10.1148/radiology.203.1.9122423. PMID:
9122423.

62. Yoshikawa J, Matsui O, Kadoya M, Gabata T, Arai K, Takashima T. Delayed enhancement of fibrotic areas in hepatic masses:
CT–pathologic correlation. J Comput Assist Tomogr. 1992; 16(2):206–211. DOI:
10.1097/00004728-199203000-00006. PMID:
1312098.
63. Valls C, Gumà A, Puig I, Sanchez A, Andía E, Serrano T, et al. Intrahepatic peripheral cholangiocarcinoma: CT
evaluation. Abdom Imaging. 2000; 25(5):490–496. DOI:
10.1007/s002610000079. PMID:
10931983.

64. Asayama Y, Yoshimitsu K, Irie H, Tajima T, Nishie A, Hirakawa M, et al. Delayed-phase dynamic CT enhancement as a prognostic factor for
mass-forming intrahepatic cholangiocarcinoma. Radiology. 2006; 238(1):150–155. DOI:
10.1148/radiol.2381041765. PMID:
16304089.

65. Jeong HT, Kim MJ, Chung YE, Choi JY, Park YN, Kim KW. Gadoxetate disodium–enhanced MRI of mass-forming
intrahepatic cholangiocarcinomas: imaging-histologic
correlation. AJR Am J Roentgenol. 2013; 201(4):W603–W611. DOI:
10.2214/AJR.12.10262. PMID:
24059399.
66. Kim SH, Lee CH, Kim BH, Kim WB, Yeom SK, Kim KA, et al. Typical and atypical imaging findings of intrahepatic
cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine
pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2012; 36(6):704–709. DOI:
10.1097/RCT.0b013e3182706562. PMID:
23192208.

68. Koh J, Chung YE, Nahm JH, Kim HY, Kim KS, Park YN, et al. Intrahepatic mass-forming cholangiocarcinoma: prognostic value of
preoperative gadoxetic acid-enhanced MRI. Eur Radiol. 2016; 26(2):407–416. DOI:
10.1007/s00330-015-3846-5. PMID:
26002136.

69. Rhee H, Choi SH, Park JH, Cho ES, Yeom SK, Park S, et al. Preoperative magnetic resonance imaging-based prognostic model
for mass-forming intrahepatic cholangiocarcinoma. Liver Int. 2022; 42(4):930–941. DOI:
10.1111/liv.15196. PMID:
35152534.

70. Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic
cholangiocarcinoma. Ann Surg Oncol. 2009; 16(1):14–22. DOI:
10.1245/s10434-008-0180-z. PMID:
18987916.

71. Raoof M, Dumitra S, Ituarte PHG, Melstrom L, Warner SG, Fong Y, et al. Development and validation of a prognostic score for intrahepatic
cholangiocarcinoma. JAMA Surg. 2017; 152(5):e170117. DOI:
10.1001/jamasurg.2017.0117. PMID:
28297009. PMCID:
PMC5624806.
72. Ji GW, Xu Q, Jiao CY, Lu M, Xu ZG, Zhang B, et al. Translating imaging traits of mass-forming intrahepatic
cholangiocarcinoma into the clinic: from prognostic to therapeutic
insights. JHEP Rep. 2023; 5(10):100839. DOI:
10.1016/j.jhepr.2023.100839. PMID:
37663120. PMCID:
PMC10468367.
73. Jiang W, Zeng ZC, Tang ZY, Fan J, Sun HC, Zhou J, et al. A prognostic scoring system based on clinical features of
intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011; 22(7):1644–1652. DOI:
10.1093/annonc/mdq650. PMID:
21212156.

74. Pandey A, Mohseni A, Shaghaghi M, Pandey P, Rezvani Habibabadi R, Hazhirkarzar B, et al. Incremental value of volumetric multiparametric MRI over Fudan
score for prognosis of unresectable intrahepatic cholangiocarcinoma treated
with systemic chemotherapy. Eur J Radiol. 2024; 170:111196. DOI:
10.1016/j.ejrad.2023.111196. PMID:
38029705.
75. Sposito C, Droz dit Busset M, Virdis M, Citterio D, Flores M, Bongini M, et al. The role of lymphadenectomy in the surgical treatment of
intrahepatic cholangiocarcinoma: a review. Eur J Surg Oncol. 2022; 48(1):150–159. DOI:
10.1016/j.ejso.2021.08.009. PMID:
34412956.

76. Sposito C, Ratti F, Cucchetti A, Ardito F, Ruzzenente A, Di Sandro S, et al. Survival benefit of adequate lymphadenectomy in patients
undergoing liver resection for clinically node-negative intrahepatic
cholangiocarcinoma. J Hepatol. 2023; 78(2):356–363. DOI:
10.1016/j.jhep.2022.10.021. PMID:
36328332.

78. Kubo S, Shinkawa H, Asaoka Y, Ioka T, Igaki H, Izumi N, et al. Liver cancer study group of Japan clinical practice guidelines
for intrahepatic cholangiocarcinoma. Liver Cancer. 2022; 11(4):290–314. DOI:
10.1159/000522403. PMID:
35978598. PMCID:
PMC9294959.

79. Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, Kendrick ML, Huebner M, Que FG. Lymphadenectomy in the staging and treatment of intrahepatic
cholangiocarcinoma: a population-based study using the National Cancer
Institute SEER database. HPB. 2011; 13(9):612–620. DOI:
10.1111/j.1477-2574.2011.00340.x. PMID:
21843261. PMCID:
PMC3183445.

80. Zhang XF, Chen Q, Kimbrough CW, Beal EW, Lv Y, Chakedis J, et al. Lymphadenectomy for intrahepatic cholangiocarcinoma: has nodal
evaluation been increasingly adopted by surgeons over time? A national
database analysis. J Gastrointest Surg. 2018; 22(4):668–675. DOI:
10.1007/s11605-017-3652-2. PMID:
29264768.

81. Mason MC, Massarweh NN, Tzeng CWD, Chiang YJ, Chun YS, Aloia TA, et al. Time to rethink upfront surgery for resectable intrahepatic
cholangiocarcinoma? Implications from the neoadjuvant
experience. Ann Surg Oncol. 2021; 28(11):6725–6735. DOI:
10.1245/s10434-020-09536-w. PMID:
33586068.

82. Utuama O, Permuth JB, Dagne G, Sanchez-Anguiano A, Alman A, Kumar A, et al. Neoadjuvant chemotherapy for intrahepatic cholangiocarcinoma: a
propensity score survival analysis supporting use in patients with high-risk
disease. Ann Surg Oncol. 2021; 28(4):1939–1949. DOI:
10.1245/s10434-020-09478-3. PMID:
33415559.

83. Zanuso V, Tesini G, Valenzi E, Rimassa L. New systemic treatment options for advanced
cholangiocarcinoma. J Liver Cancer. 2024; 24(2):155–170. DOI:
10.17998/jlc.2024.08.07. PMID:
39113642. PMCID:
PMC11449581.
84. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory
cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind,
placebo-controlled, phase 3 study. Lancet Oncol. 2020; 21(6):796–807. DOI:
10.1016/S1470-2045(20)30157-1. PMID:
32416072.

85. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for
patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3
randomized clinical ClarIDHy trial. JAMA Oncol. 2021; 7(11):1669–1677. DOI:
10.1001/jamaoncol.2021.3836. PMID:
34554208. PMCID:
PMC8461552.

86. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic
cholangiocarcinoma. N Engl J Med. 2023; 388(3):228–239. DOI:
10.1056/NEJMoa2206834. PMID:
36652354.

87. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or
metastatic cholangiocarcinoma: a multicentre, open-label, phase 2
study. Lancet Oncol. 2020; 21(5):671–684. DOI:
10.1016/S1470-2045(20)30109-1. PMID:
32203698.

88. Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch
repair deficient cancers: updated analysis from the phase II KEYNOTE-158
study. Ann Oncol. 2022; 33(9):929–938. DOI:
10.1016/j.annonc.2022.05.519. PMID:
35680043.

89. Zhu Y, Chen J, Kong W, Mao L, Kong W, Zhou Q, et al. Predicting IDH mutation status of intrahepatic
cholangiocarcinomas based on contrast-enhanced CT features. Eur Radiol. 2018; 28(1):159–169. DOI:
10.1007/s00330-017-4957-y. PMID:
28752218.

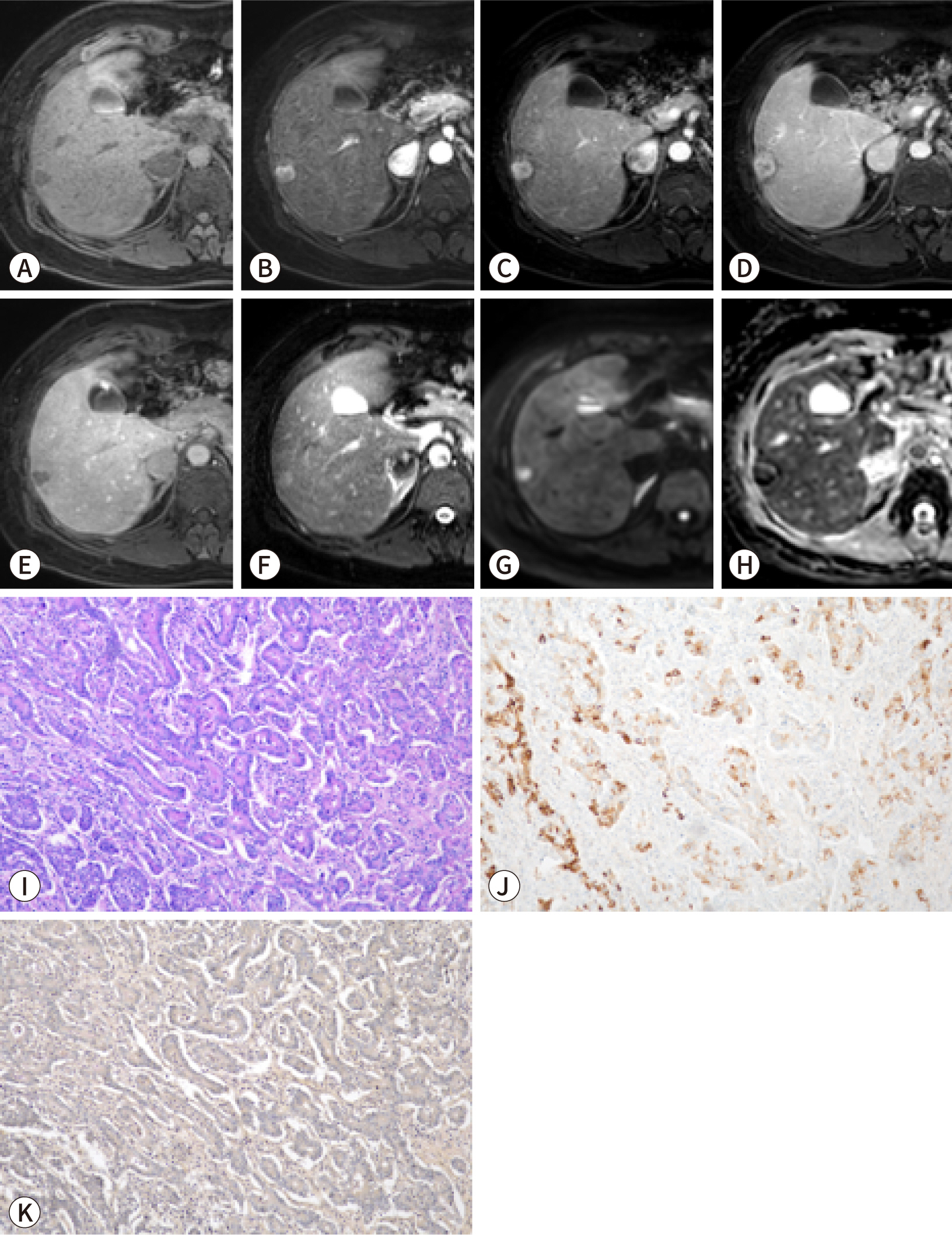
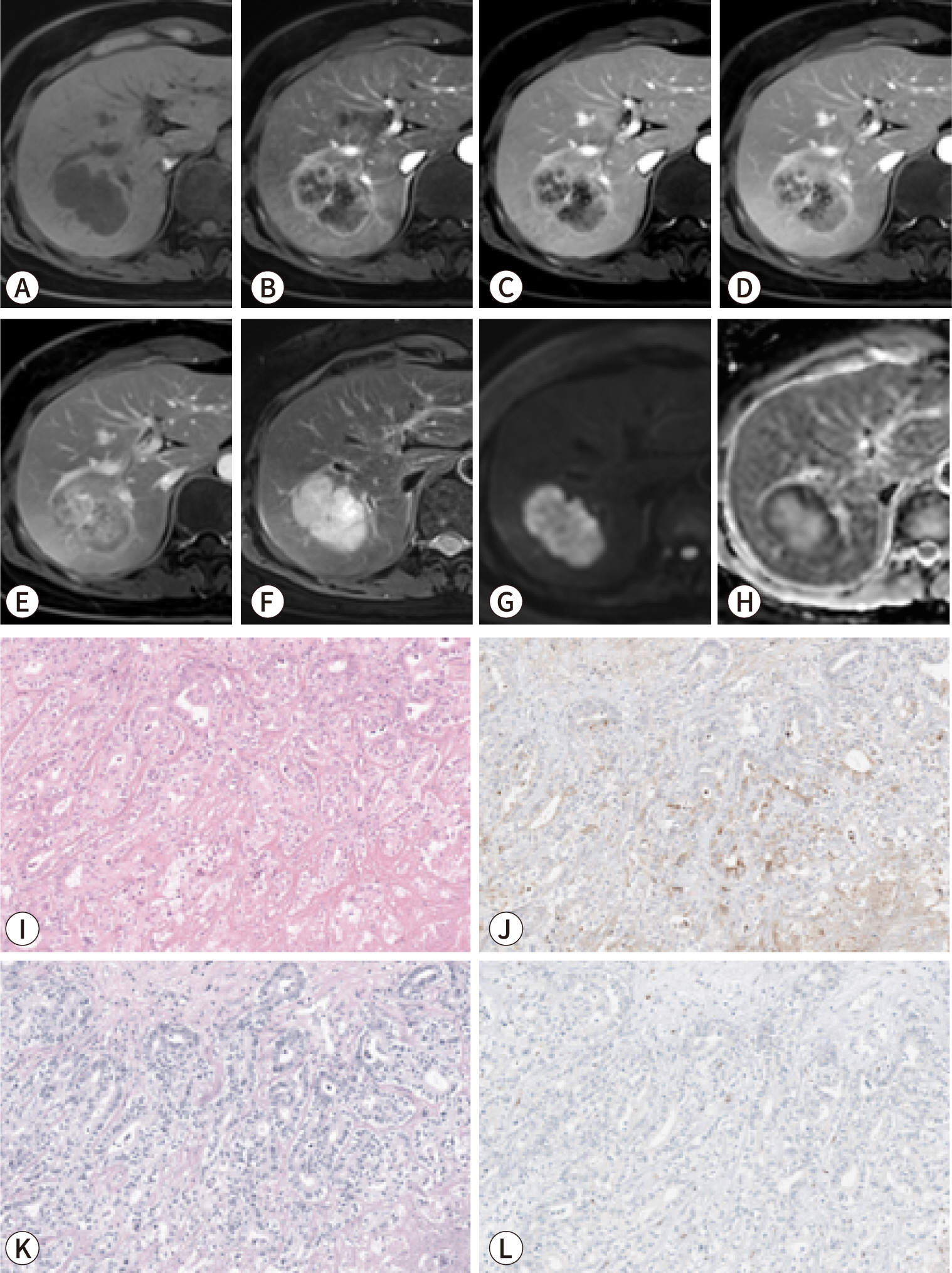
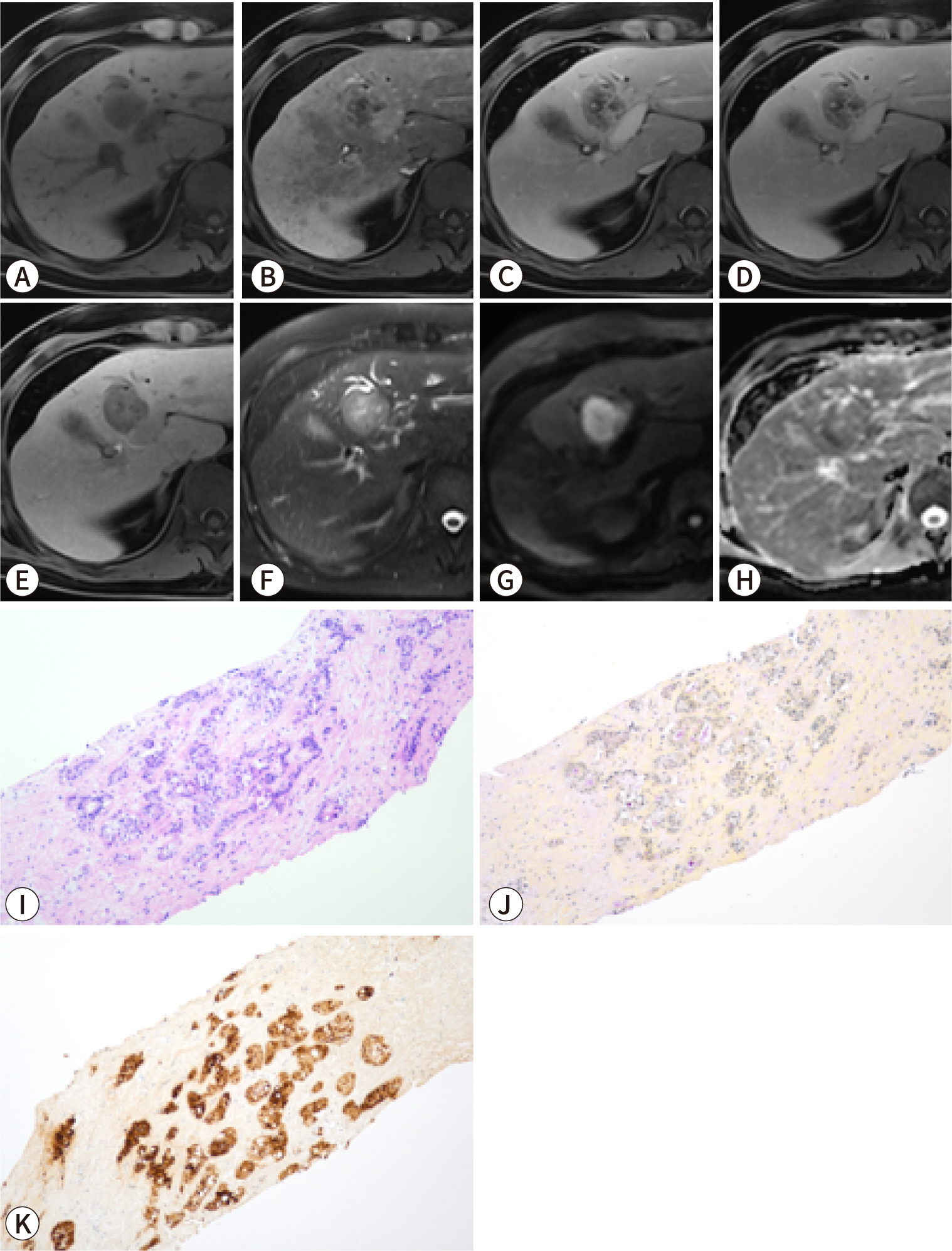
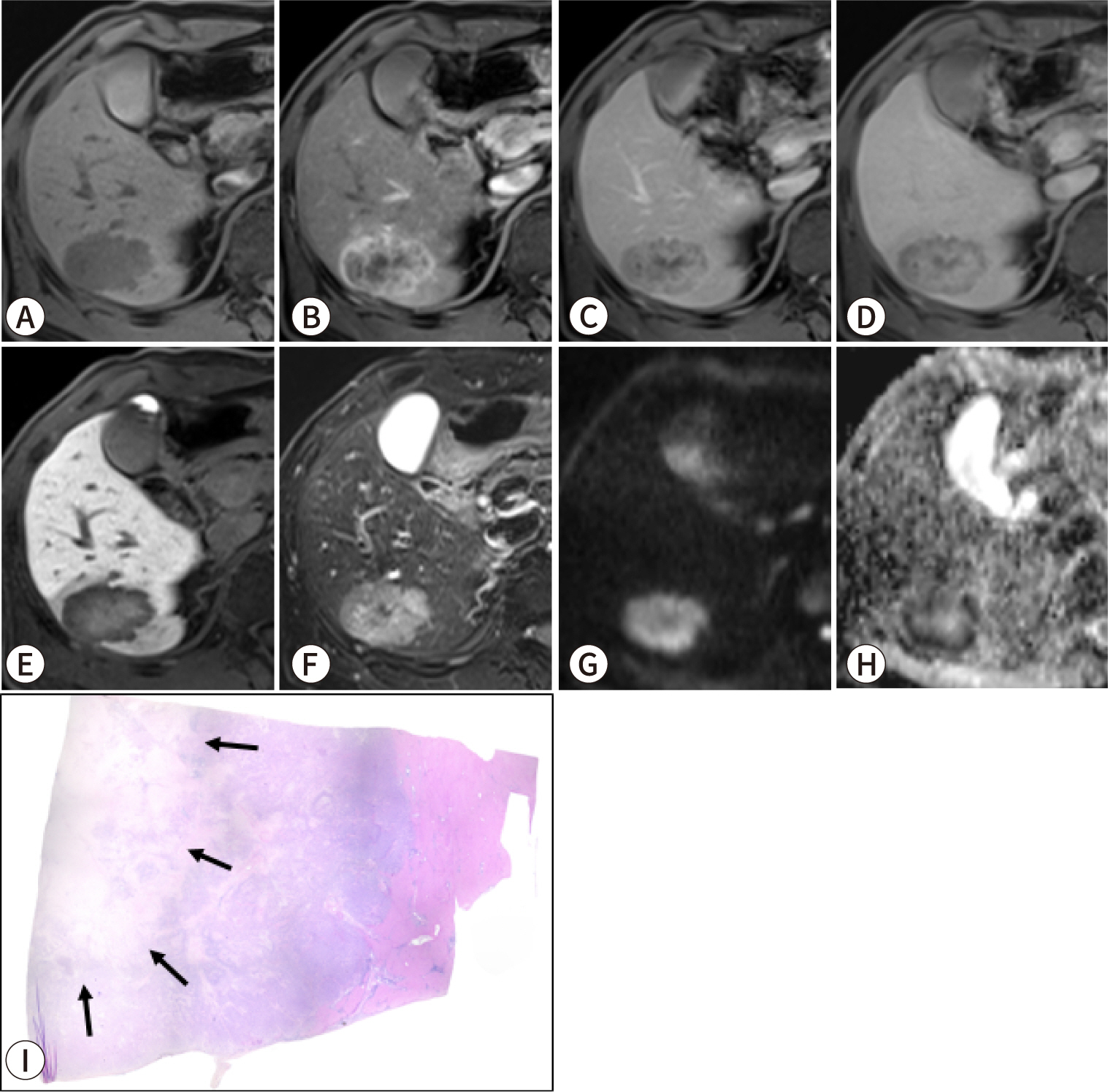




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download