1. Chun SG, Hu C, Komaki RU, Timmerman RD, Schild SE, Bogart JA, et al. Long-term prospective outcomes of intensity modulated
radiotherapy for locally advanced lung cancer: a secondary analysis of a
randomized clinical trial. JAMA Oncol. 2024; 10(8):1111–1115. DOI:
10.1001/jamaoncol.2024.1841. PMID:
38935373.

2. Fathy MM, Hassan BZ, El-Gebaly RH, Mokhtar MH. Dosimetric evaluation study of IMRT and VMAT techniques for
prostate cancer based on different multileaf collimator
designs. Radiat Environ Biophys. 2023; 62(1):97–106. DOI:
10.1007/s00411-022-01011-2. PMID:
36576578. PMCID:
PMC9950215.

3. Mohan G, Ayisha Hamna TP, Jijo AJ, Saradha Devi KM, Narayanasamy A, Vellingiri B. Recent advances in radiotherapy and its associated side effects
in cancer: a review. J Basic Appl Zool. 2019; 80(1):1–10. DOI:
10.1186/s41936-019-0083-5.
4. Zaorsky NG, Harrison AS, Trabulsi EJ, Gomella LG, Showalter TN, Hurwitz MD, et al. Evolution of advanced technologies in prostate cancer
radiotherapy. Nat Rev Urol. 2013; 10(10):565–579. DOI:
10.1038/nrurol.2013.185. PMID:
24018567.

5. Lim DH. Localized intracranial germinoma: is it time to re-define target
volume for whole ventricular irradiation? Radiat Oncol J. 2023; 41(2):59–60. DOI:
10.3857/roj.2023.00423. PMID:
37403347. PMCID:
PMC10326506.

6. Yu JI. Myxoid liposarcoma: a well-defined clinical target variant in
radiotherapy for soft tissue sarcoma. Radiat Oncol J. 2022; 40(4):213–215. DOI:
10.3857/roj.2022.00598. PMID:
36606298. PMCID:
PMC9830037.

7. Teh BS, Woo SY, Butler EB. Intensity modulated radiation therapy (IMRT): a new promising
technology in radiation oncology. Oncol. 1999; 4(6):433–442. DOI:
10.1634/theoncologist.4-6-433.

8. Scaringi C, Agolli L, Minniti G. Technical advances in radiation therapy for brain
tumors. Anticancer Res. 2018; 38(11):6041–6045. DOI:
10.21873/anticanres.12954. PMID:
30396918.

9. Park SY, Kim J, Chun M, Ahn H, Park JM. Assessment of the modulation degrees of intensity-modulated
radiation therapy plans. Radiat Oncol. 2018; 13:1–8. DOI:
10.1186/s13014-018-1193-9. PMID:
30545396. PMCID:
PMC6293636.
10. Tanaka Y, Hashimoto M, Ishigami M, Nakano M, Hasegawa T. Development of a novel delivery quality assurance system based on
simultaneous verification of dose distribution and binary multi-leaf
collimator opening in helical tomotherapy. Radiat Oncol. 2023; 18(1):180. DOI:
10.1186/s13014-023-02366-6. PMID:
37919745. PMCID:
PMC10621123.
11. Mandava A, Koppula V, Kandati M, Raju KVVN. Synchronous radiation-induced enterovesical and enterocervical
fistulas in carcinoma of the uterine cervix. Radiat Oncol J. 2023; 41(4):297–300. DOI:
10.3857/roj.2023.00500. PMID:
38185935. PMCID:
PMC10772593.

12. Kavanagh BD, Timmerman RD. Stereotactic radiosurgery and stereotactic body radiation
therapy: an overview of technical considerations and clinical
applications. Hematol Oncol Clin. 2006; 20(1):87–95. DOI:
10.1016/j.hoc.2006.01.009. PMID:
16580558.

13. Jia-Mahasap B, Madla C, Sripan P, Chitapanarux I, Tharavichitkul E, Chakrabandhu S, et al. Stereotactic radiosurgery for limited brain metastasis using
three different techniques: helical tomotherapy, volumetric modulated arc
therapy, and cone-based LINAC radiosurgery. Radiat Oncol J. 2022; 40(4):232–241. DOI:
10.3857/roj.2022.00136. PMID:
36606300. PMCID:
PMC9830036.

14. Jaffray DA, Siewerdsen JH. Cone-beam computed tomography with a flat-panel imager: initial
performance characterization. Med Phys. 2000; 27(6):1311–1323. DOI:
10.1118/1.599009. PMID:
10902561.

15. Balter JM, Kessler ML. Imaging and alignment for image-guided radiation
therapy. J Clin Oncol. 2007; 25(8):931–937. DOI:
10.1200/JCO.2006.09.7998. PMID:
17350941.
16. Kim J, Lee H, Wu HG, Chie EK, Kang HC, Park JM. Development of patient-controlled respiratory gating system based
on visual guidance for magnetic-resonance image-guided radiation
therapy. Med Phys. 2017; 44(9):4838–4846. DOI:
10.1002/mp.12447. PMID:
28675492.

17. Korreman S, Eriksen JG, Grau C. The changing role of radiation oncology professionals in a world
of AI–just jobs lost–or a solution to the under-provision of
radiotherapy? Clin Transl Radiat Oncol. 2021; 26:104–107. DOI:
10.1016/j.ctro.2020.04.012. PMID:
33364449. PMCID:
PMC7752957.

18. Yang W, Williams JH, Hogan PF, Bruinooge SS, Rodriguez GI, Kosty MP, et al. Projected supply of and demand for oncologists and radiation
oncologists through 2025: an aging, better-insured population will result in
shortage. J Oncol Pract. 2014; 10(1):39–45. DOI:
10.1200/JOP.2013.001319. PMID:
24443733.

19. Kim TH. Has the growing evidence of radiotherapy for hepatocellular
carcinoma increased the use of radiotherapy in elderly
patients? Radiat Oncol J. 2023; 41(3):141–143. DOI:
10.3857/roj.2023.00710. PMID:
37793622. PMCID:
PMC10556838.

20. Orszag PR. US health care reform: cost containment and improvement in
quality. JAMA. 2016; 316(5):493–495. DOI:
10.1001/jama.2016.9876. PMID:
27400156.
22. Chakravarty K, Antontsev V, Bundey Y, Varshney J. Driving success in personalized medicine through AI-enabled
computational modeling. Drug Discov Today. 2021; 26(6):1459–1465. DOI:
10.1016/j.drudis.2021.02.007. PMID:
33609781.

23. Weerarathna IN, Kamble AR, Luharia A. Artificial intelligence applications for biomedical cancer
research: a review. Cureus. 2023; 15(11):e48307. DOI:
10.7759/cureus.48307.
24. Yoo S, Sheng Y, Blitzblau R, McDuff S, Champ C, Morrison J, et al. Clinical experience with machine learning-based automated
treatment planning for whole breast radiation therapy. Adv Radiat Oncol. 2021; 6(2):100656. DOI:
10.1016/j.adro.2021.100656. PMID:
33748540. PMCID:
PMC7966969.
25. Wang C, Zhu X, Hong JC, Zheng D. Artificial intelligence in radiotherapy treatment planning:
present and future. Technol Cancer Res Treat. 2019; 18:1533033819873922. DOI:
10.1177/1533033819873922. PMID:
31495281. PMCID:
PMC6732844.
26. Kiser KJ, Fuller CD, Reed VK. Artificial intelligence in radiation oncology treatment planning:
a brief overview. J Med Artif Intell. 2019; 2:9. DOI:
10.21037/jmai.2019.04.02.

27. Blumenfeld P, Arbit E, Den R, Salhab A, Falick Michaeli T, Wygoda M, et al. Real world clinical experience using daily intelligence-assisted
online adaptive radiotherapy for head and neck cancer. Radiat Oncol. 2024; 19(1):43. DOI:
10.1186/s13014-024-02436-3. PMID:
38555453. PMCID:
PMC10981810.
28. Wang W, Sheng Y, Wang C, Zhang J, Li X, Palta M, et al. Fluence map prediction using deep learning models – direct
plan generation for pancreas stereotactic body radiation
therapy. Front Artif Intell. 2020; 3:68. DOI:
10.3389/frai.2020.00068. PMID:
33733185. PMCID:
PMC7861344.
29. Cai W, Ding S, Li H, Zhou X, Dou W, Zhou L, et al. Automatic IMRT treatment planning through fluence prediction and
plan fine-tuning for nasopharyngeal carcinoma. Radiat Oncol. 2024; 19(1):39. DOI:
10.1186/s13014-024-02401-0. PMID:
38509540. PMCID:
PMC10956235.
30. Byrne M, Archibald-Heeren B, Hu Y, Teh A, Beserminji R, Cai E, et al. Varian ethos online adaptive radiotherapy for prostate cancer:
early results of contouring accuracy, treatment plan quality, and treatment
time. J Appl Clin Med Phys. 2022; 23(1):e13479. DOI:
10.1002/acm2.13479. PMID:
34846098. PMCID:
PMC8803282.

31. Archambault Y, Boylan C, Bullock D, Morgas T, Peltola J, Ruokokoski E, et al. Making on-line adaptive radiotherapy possible using artificial
intelligence and machine learning for efficient daily
re-planning. Med Phys Int J. 2020; 8(2):77–86.
32. Massat MB. Oncology EMRs: more than a patient record. Appl Radiat Oncol. 2016; (3):38–40. DOI:
10.37549/ARO1105.

33. Rayed ME, Islam SMS, Niha SI, Jim JR, Kabir MM, Mridha MF. Deep learning for medical image segmentation: state-of-the-art
advancements and challenges. Inform Med Unlocked. 2024; 47:101504. DOI:
10.1016/j.imu.2024.101504.
34. Hooshangnejad H, Feng X, Huang G, Zhang R, Kelly K, Chen Q, et al. EXACT-Net: EHR-guided lung tumor auto-segmentation for non-small cell
lung cancer radiotherapy. arXiv: 2402.14099 [Preprint]. 2024.
35. Atiya SU, Ramesh NVK. Enhancing non-small cell lung cancer radiotherapy planning: a
deep learning-based multi-modal fusion approach for accurate GTV
segmentation. Biomed Signal Process Control. 2024; 92:105987. DOI:
10.1016/j.bspc.2024.105987.
36. Rai HM. Cancer detection and segmentation using machine learning and deep
learning techniques: a review. Multimed Tools Appl. 2024; 83(9):27001–27035. DOI:
10.1007/s11042-023-16520-5.

37. Abo-El-Rejal A, Ayman SE, Aymen F. Advances in breast cancer segmentation: a comprehensive
review. Acadlore Trans AI Mach Learn. 2024; 3(2):70–83. DOI:
10.56578/ataiml030201.
38. Liu X, Qu L, Xie Z, Zhao J, Shi Y, Song Z. Towards more precise automatic analysis: a systematic review of
deep learning-based multi-organ segmentation. BioMed Eng OnLine. 2024; 23(1):52. DOI:
10.1186/s12938-024-01238-8. PMID:
38851691. PMCID:
PMC11162022.
39. Zi Y, Wang Q, Gao Z, Cheng X, Mei T. Research on the application of deep learning in medical image
segmentation and 3D reconstruction. Acad J Sci Technol. 2024; 10(2):8–12. DOI:
10.54097/0h77ge77.

40. Wang TW, Hong JS, Chiu HY, Chao HS, Chen YM, Wu YT. Standalone deep learning versus experts for diagnosis lung cancer
on chest computed tomography: a systematic review. Eur Radiol. 2024; May. 22. [Epub]. DOI:
10.1007/s00330-024-10804-6. PMID:
38777902. PMCID:
PMC11519296.
41. Santhakumar G, Takale DG, Tyagi S, Anitha R, Tiwari M, Dhanraj JA. Analysis of multimodality fusion of medical image segmentation
employing deep learning. In:. Joshi K, Kumar Gupta S, editors. editors. Human cancer diagnosis and detection using exascale computing. Beverly: Scrivener;2024. p. p. 171–183. DOI:
10.1002/9781394197705.ch11.

42. Wu Y, Luo X, Xu Z, Guo X, Ju L, Ge Z, et al. Diversified and personalized multi-rater medical image
segmentation. arXiv: 2403.13417 [Preprint]. 2024; DOI:
10.1109/CVPR52733.2024.01090.
43. Yu L, Min W, Wang S. Boundary-aware gradient operator network for medical image
segmentation. IEEE J Biomed Health Inform. 2024; 28(8):4711–4723. DOI:
10.1109/JBHI.2024.3404273. PMID:
38776204.
45. Kehayias CE, Yan Y, Bontempi D, Quirk S, Bitterman DS, Bredfeldt JS, et al. Prospective deployment of an automated implementation solution
for artificial intelligence translation to clinical radiation
oncology. Front Oncol. 2024; 13:1305511. DOI:
10.3389/fonc.2023.1305511. PMID:
38239639. PMCID:
PMC10794768.
46. Rayn K, Gokhroo G, Jeffers B, Gupta V, Chaudhari S, Clark R, et al. Multicenter study of pelvic nodal autosegmentation algorithm of
Siemens Healthineers: comparison of male versus female
pelvis. Adv Radiat Oncol. 2024; 9(2):101326. DOI:
10.1016/j.adro.2023.101326. PMID:
38405314. PMCID:
PMC10885554.
47. Schwartz DL, Dong L. Adaptive radiation therapy for head and neck cancer: can an old
goal evolve into a new standard? J Oncol. 2011; 2011(1):690595. DOI:
10.1155/2011/690595. PMID:
20847944. PMCID:
PMC2933914.
48. Glide-Hurst CK, Lee P, Yock AD, Olsen JR, Cao M, Siddiqui F, et al. Adaptive radiation therapy (ART) strategies and technical
considerations: a state of the ART review from NRG oncology. Int J Radiat Oncol Biol Phys. 2021; 109(4):1054–1075. DOI:
10.1016/j.ijrobp.2020.10.021. PMID:
33470210. PMCID:
PMC8290862.

49. Liu H, Schaal D, Curry H, Clark R, Magliari A, Kupelian P, et al. Review of cone beam computed tomography based online adaptive
radiotherapy: current trend and future direction. Radiat Oncol. 2023; 18(1):144. DOI:
10.1186/s13014-023-02340-2. PMID:
37660057. PMCID:
PMC10475190.
50. Wang YF, Price MJ, Elliston CD, Munbodh R, Spina CS, Horowitz DP, et al. Enhancing safety in AI-driven cone beam CT-based online adaptive
radiation therapy: development and implementation of an interdisciplinary
workflow. Adv Radiat Oncol. 2024; 9(3):101399. DOI:
10.1016/j.adro.2023.101399. PMID:
38292890. PMCID:
PMC10823112.
51. Sibolt P, Andersson LM, Calmels L, Sjöström D, Bjelkengren U, Geertsen P, et al. Clinical implementation of artificial intelligence-driven
cone-beam computed tomography-guided online adaptive radiotherapy in the
pelvic region. Phys Imaging Radiat Oncol. 2021; 17:1–7. DOI:
10.1016/j.phro.2020.12.004. PMID:
33898770. PMCID:
PMC8057957.
52. Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM, et al. Adaptive radiotherapy: the Elekta Unity MR-linac
concept. Clin Transl Radiat Oncol. 2019; 18:54–59. DOI:
10.1016/j.ctro.2019.04.001. PMID:
31341976. PMCID:
PMC6630157.
53. Badawy M, Ramadan N, Hefny HA. Healthcare predictive analytics using machine learning and deep
learning techniques: a survey. J Electr Syst Inf Technol. 2023; 10(1):40. DOI:
10.1186/s43067-023-00108-y.
54. Lee Y, Choi HJ, Kim H, Kim S, Kim MS, Cha H, et al. Feasibility of artificial intelligence-driven interfractional
monitoring of organ changes by mega-voltage computed tomography in
intensity-modulated radiotherapy of prostate cancer. Radiat Oncol J. 2023; 41(3):186–198. DOI:
10.3857/roj.2023.00444. PMID:
37793628. PMCID:
PMC10556843.

55. Mijderwijk HJ, Steiger HJ. Predictive analytics in clinical practice: advantages and
disadvantages. In:. Staartjes VE, Regli L, Serra C, editors. editors. Machine learning in clinical neuroscience: foundations and
applications. Cham: Springer;2022. p. p. 263–268. DOI:
10.1007/978-3-030-85292-4_30. PMID:
34862550.
56. Huang Y, Li J, Li M, Aparasu RR. Application of machine learning in predicting survival outcomes
involving real-world data: a scoping review. BMC Med Res Methodol. 2023; 23(1):268. DOI:
10.1186/s12874-023-02078-1. PMID:
37957593. PMCID:
PMC10641971.
57. Somashekhar SP, Sepúlveda MJ, Puglielli S, Norden AD, Shortliffe EH, Kumar CR, et al. Watson for oncology and breast cancer treatment recommendations:
agreement with an expert multidisciplinary tumor board. Ann Oncol. 2018; 29(2):418–423. DOI:
10.1093/annonc/mdx781. PMID:
29324970.

58. Tsang DS, Tsui G, Santiago AT, Keller H, Purdie T, Mcintosh C, et al. A prospective study of machine learning-assisted radiation
therapy planning for patients receiving 54 Gy to the brain. Int J Radiat Oncol Biol Phys. 2024; 119(5):1429–1436. DOI:
10.1016/j.ijrobp.2024.02.022. PMID:
38432285.

59. Budach L, Feuerpfeil M, Ihde N, Nathansen A, Noack N, Patzlaff H, et al. The effects of data quality on machine learning performance. arXiv: 2207.14529 [Preprint]. 2022.
60. Whang SE, Roh Y, Song H, Lee JG. Data collection and quality challenges in deep learning: a
data-centric AI perspective. VLDB J. 2023; 32(4):791–813. DOI:
10.1007/s00778-022-00775-9.

61. Aldoseri A, Al-Khalifa KN, Hamouda AM. Re-thinking data strategy and integration for artificial
intelligence: concepts, opportunities, and challenges. Appl Sci. 2023; 13(12):7082. DOI:
10.3390/app13127082.

62. Choi HS, Song JY, Shin KH, Chang JH, Jang BS. Developing prompts from large language model for extracting
clinical information from pathology and ultrasound reports in breast
cancer. Radiat Oncol J. 2023; 41(3):209–216. DOI:
10.3857/roj.2023.00633. PMID:
37793630. PMCID:
PMC10556835.

63. Arasteh ST, Lotfinia M, Nolte T, Saehn M, Isfort P, Kuhl C, et al. Preserving privacy in domain transfer of medical AI models comes at no
performance costs: the integral role of differential privacy. arXiv: 2306.06503 [Preprint]. 2023.
64. Kessel KA, Combs SE. Data management, documentation and analysis systems in radiation
oncology: a multi-institutional survey. Radiat Oncol. 2015; 10:1–6. DOI:
10.1186/s13014-015-0543-0. PMID:
26572494. PMCID:
PMC4647666.
65. Hughes N, Kalra D. Data standards and platform interoperability. In:. He W, Fang Y, Wang H, editors. editors. Real-world evidence in medical product development. Cham: Springer;2023. p. p. 79–107. DOI:
10.1007/978-3-031-26328-6_6. PMCID:
PMC10098370.

66. Bukowski M, Farkas R, Beyan O, Moll L, Hahn H, Kiessling F, et al. Implementation of eHealth and AI integrated diagnostics with
multidisciplinary digitized data: are we ready from an international
perspective? Eur Radiol. 2020; 30:5510–5524. DOI:
10.1007/s00330-020-06874-x. PMID:
32377810. PMCID:
PMC7476980.

67. Caffery LJ, Rotemberg V, Weber J, Soyer HP, Malvehy J, Clunie D. The role of DICOM in artificial intelligence for skin
disease. Front Med. 2021; 7:619787. DOI:
10.3389/fmed.2020.619787. PMID:
33644087. PMCID:
PMC7902872.
69. Pesapane F, Volonté C, Codari M, Sardanelli F. Artificial intelligence as a medical device in radiology: ethical
and regulatory issues in Europe and the United States. Insights Imaging. 2018; 9:745–753. DOI:
10.1007/s13244-018-0645-y. PMID:
30112675. PMCID:
PMC6206380.

70. Hasan HE, Jaber D, Khabour OF, Alzoubi KH. Ethical considerations and concerns in the implementation of AI
in pharmacy practice: a cross-sectional study. BMC Med Ethics. 2024; 25(1):55. DOI:
10.1186/s12910-024-01062-8. PMID:
38750441. PMCID:
PMC11096093.
71. Mohammad Amini M, Jesus M, Fanaei Sheikholeslami D, Alves P, Hassanzadeh Benam A, Hariri F. Artificial intelligence ethics and challenges in healthcare
applications: a comprehensive review in the context of the European GDPR
mandate. Mach Learn Knowl Extr. 2023; 5(3):1023–1035. DOI:
10.3390/make5030053.

72. Duong MT, Rauschecker AM, Rudie JD, Chen PH, Cook TS, Bryan RN, et al. Artificial intelligence for precision education in
radiology. Br J Radiol. 2019; 92(1103):20190389. DOI:
10.1259/bjr.20190389. PMID:
31322909. PMCID:
PMC6849670.
73. Talwar S, Dhir A, Islam N, Kaur P, Almusharraf A. Resistance of multiple stakeholders to e-health innovations:
integration of fundamental insights and guiding research
paths. J Bus Res. 2023; 166:114135. DOI:
10.1016/j.jbusres.2023.114135.
74. van de Sande D, Van Genderen ME, Smit JM, Huiskens J, Visser JJ, Veen RER, et al. Developing, implementing and governing artificial intelligence in
medicine: a step-by-step approach to prevent an artificial intelligence
winter. BMJ Health Care Inform. 2022; 29(1):e100495. DOI:
10.1136/bmjhci-2021-100495. PMID:
35185012. PMCID:
PMC8860016.
75. Brady AP, Allen B, Chong J, Kotter E, Kottler N, Mongan J, et al. Developing, purchasing, implementing and monitoring AI tools in
radiology: practical considerations. A multi-society statement from the ACR,
CAR, ESR, RANZCR & RSNA. Can Assoc Radiol J. 2024; 75(2):226–244. DOI:
10.1177/08465371231222229. PMID:
38251882.

76. Huynh E, Hosny A, Guthier C, Bitterman DS, Petit SF, Haas-Kogan DA, et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020; 17(12):771–781. DOI:
10.1038/s41571-020-0417-8. PMID:
32843739.

77. Kawamura M, Kamomae T, Yanagawa M, Kamagata K, Fujita S, Ueda D, et al. Revolutionizing radiation therapy: the role of AI in clinical
practice. J Radiat Res. 2024; 65(1):1–9. DOI:
10.1093/jrr/rrad090. PMID:
37996085. PMCID:
PMC10803173.
78. Cui S, Ten Haken RK, El Naqa I. Integrating multiomics information in deep learning architectures
for joint actuarial outcome prediction in non-small cell lung cancer
patients after radiation therapy. Int J Radiat Oncol Biol Phys. 2021; 110(3):893–904. DOI:
10.1016/j.ijrobp.2021.01.042. PMID:
33539966. PMCID:
PMC8180510.

79. Grass GD, Mills MN, Scott JG, Eschrich SA, Torres-Roca J. Genomics and radiomics: tools to see the unseen to personalize
radiation therapy. Appl Radiat Oncol. 2019; 8:9–22. DOI:
10.37549/ARO1213.
80. McGregor BA, Vidal GA, Shah SA, Mitchell JD, Hendifar AE. Remote oncology care: review of current technology and future
directions. Cureus. 2020; 12(8):e10156. DOI:
10.7759/cureus.10156.
81. Harvey H, Glocker B. A standardised approach for preparing imaging data for machine
learning tasks in radiology. In:. Ranschaert ER, Morozov S, Algra PR, editors. editors. Artificial intelligence in medical imaging: opportunities, applications
and risks. Cham: Springer;2019. p. p. 61–72. DOI:
10.1007/978-3-319-94878-2_6.

82. Wahid KA, Glerean E, Sahlsten J, Jaskari J, Kaski K, Naser MA, et al. Artificial intelligence for radiation oncology applications using
public datasets. Semin Radiat Oncol. 2022; 32(4):400–414. DOI:
10.1016/j.semradonc.2022.06.009. PMID:
36202442. PMCID:
PMC9587532.

83. Fraser AG, Biasin E, Bijnens B, Bruining N, Caiani EG, Cobbaert K, et al. Artificial intelligence in medical device software and high-risk
medical devices: a review of definitions, expert recommendations and
regulatory initiatives. Expert Rev Med Devices. 2023; 20(6):467–491. DOI:
10.1080/17434440.2023.2184685. PMID:
37157833.

84. Beckers R, Kwade Z, Zanca F. The EU medical device regulation: implications for artificial
intelligence-based medical device software in medical
physics. Phys Med. 2021; 83:1–8. DOI:
10.1016/j.ejmp.2021.02.011. PMID:
33657513.

85. Amann J, Blasimme A, Vayena E, Frey D, Madai VI. Explainability for artificial intelligence in healthcare: a
multidisciplinary perspective. BMC Med Inform Decis Mak. 2020; 20:310. DOI:
10.1186/s12911-020-01332-6. PMID:
33256715. PMCID:
PMC7706019.
86. Dwivedi YK, Hughes L, Ismagilova E, Aarts G, Coombs C, Crick T, et al. Artificial intelligence (AI): multidisciplinary perspectives on
emerging challenges, opportunities, and agenda for research, practice and
policy. Int J Inf Manag. 2021; 57:101994. DOI:
10.1016/j.ijinfomgt.2019.08.002.
87. Mauthner NS, Parry O. Open access digital data sharing: principles, policies and
practices. Soc Epistemol. 2013; 27(1):47–67. DOI:
10.1080/02691728.2012.760663.
88. Knapič S, Malhi A, Saluja R, Främling K. Explainable artificial intelligence for human decision support
system in the medical domain. Mach Learn Knowl Extr. 2021; 3(3):740–770. DOI:
10.3390/make3030037.

89. Band SS, Yarahmadi A, Hsu CC, Biyari M, Sookhak M, Ameri R, et al. Application of explainable artificial intelligence in medical
health: a systematic review of interpretability methods. Inform Med Unlocked. 2023; 40:101286. DOI:
10.1016/j.imu.2023.101286.
90. Mensah GB. Artificial intelligence and ethics: a comprehensive review of
bias mitigation, transparency, and accountability in AI
Systems. ResearchGate. [Preprint]. 2023.
91. Khanna S, Srivastava S. Patient-centric ethical frameworks for privacy, transparency, and
bias awareness in deep learning-based medical systems. Appl Res Artif Intell Cloud Comput. 2020; 3(1):16–35.
92. Vaassen F, Hazelaar C, Vaniqui A, Gooding M, van der Heyden B, Canters R, et al. Evaluation of measures for assessing time-saving of automatic
organ-at-risk segmentation in radiotherapy. Phys Imaging Radiat Oncol. 2020; 13:1–6. DOI:
10.1016/j.phro.2019.12.001. PMID:
33458300. PMCID:
PMC7807544.

93. Habuza T, Navaz AN, Hashim F, Alnajjar F, Zaki N, Serhani MA, et al. AI applications in robotics, diagnostic image analysis and
precision medicine: current limitations, future trends, guidelines on CAD
systems for medicine. Inform Med Unlocked. 2021; 24:100596. DOI:
10.1016/j.imu.2021.100596.
94. Bates DW, Saria S, Ohno-Machado L, Shah A, Escobar G. Big data in health care: using analytics to identify and manage
high-risk and high-cost patients. Health Aff. 2014; 33(7):1123–1131. DOI:
10.1377/hlthaff.2014.0041. PMID:
25006137.

95. Shah V. AI in mental health: predictive analytics and intervention
strategies. J Environ Sci Technol. 2022; 1(2):55–74.
96. Kim N, Chun J, Chang JS, Lee CG, Keum KC, Kim JS. Feasibility of continual deep learning-based segmentation for
personalized adaptive radiation therapy in head and neck
area. Cancers. 2021; 13(4):702. DOI:
10.3390/cancers13040702. PMID:
33572310. PMCID:
PMC7915955.

97. van Leeuwen KG, de Rooij M, Schalekamp S, van Ginneken B, Rutten MJCM. How does artificial intelligence in radiology improve efficiency
and health outcomes? Pediatr Radiol. 2022; 52:2087–2093. DOI:
10.1007/s00247-021-05114-8. PMID:
34117522. PMCID:
PMC9537124.

99. Kalweit G, Valiña LG, Mastroleo I, Klett A, Boedecker J, Mertelsmann R, et al. AI as an always-available oncologist: a vision for AI-optimized
cancer therapy based on real-time adaptive dosing at the patient
level. J Sci Humanit Arts. 2024; 11(1):1–12. DOI:
10.17160/josha.11.1.975.
100. Ahmed A, Aziz S, Abd-alrazaq A, Farooq F, Sheikh J. Overview of artificial intelligence–driven wearable
devices for diabetes: scoping review. J Med Internet Res. 2022; 24(8):e36010. DOI:
10.2196/36010. PMID:
35943772. PMCID:
PMC9399882.
101. Johnson KB, Wei-Qi W, Weeraratne D, Frisse ME, Misulis K, Rhee K, et al. Precision medicine, AI, and the future of personalized health
care. Clin Transl Sci. 2021; 14(1):86–93. DOI:
10.1111/cts.12884. PMID:
32961010. PMCID:
PMC7877825.
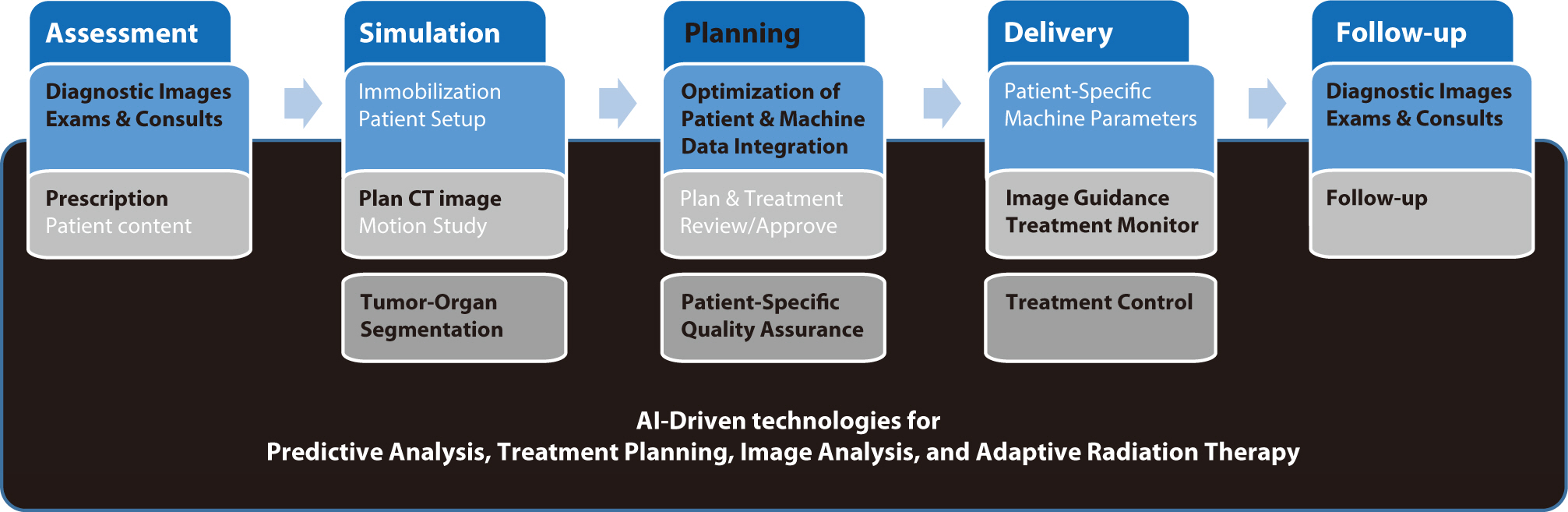
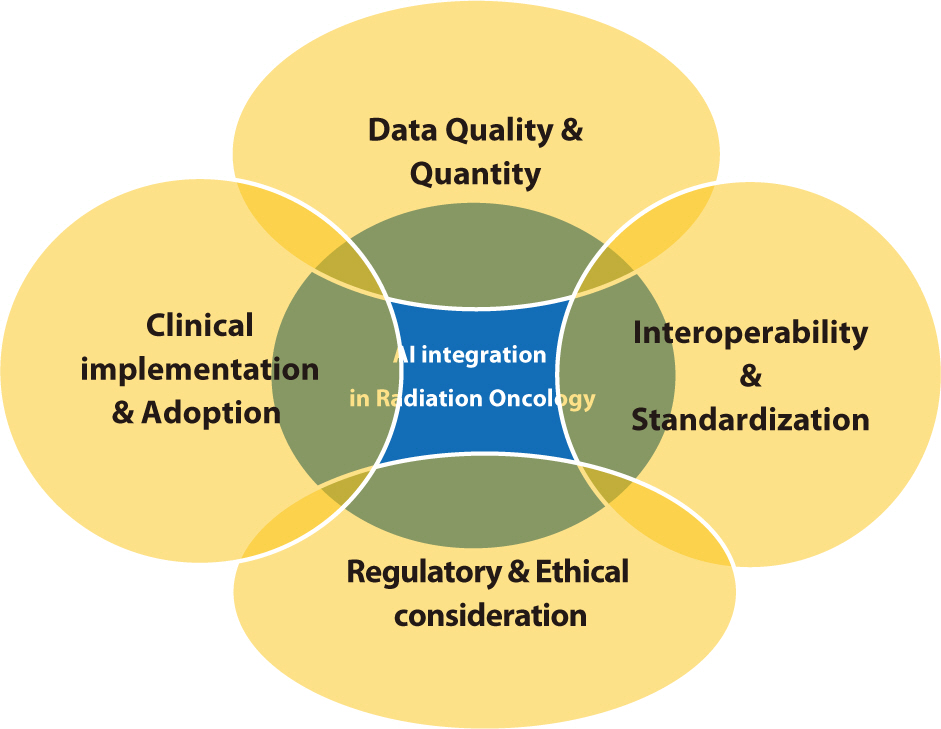
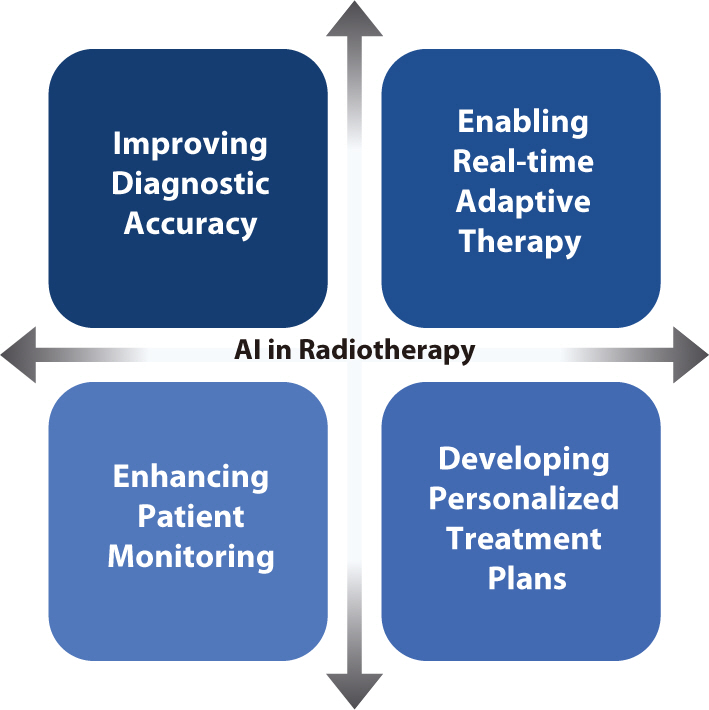




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download