Abstract
In pediatric anesthesia, respiratory adverse events often occur during emergence from anesthesia and at the time of endotracheal tube or supraglottic device removal. The removal of airway devices and extubation are conducted either while patients are deeply anesthetized or when patients awaken from anesthesia and have regained consciousness. The airways of children are easily irritated by external stimuli and are structurally prone to collapse, and the timing of both methods of airway device removal is similarly associated with various airway complications, including upper airway obstruction, coughing, or serious adverse events such as laryngospasm and desaturation. In current pediatric anesthesia practice, the choice of the timing and method of extubation is made by anesthesiologists. To achieve a smooth and safe recovery from anesthesia, understanding the unique characteristics of pediatric airways and the factors likely to contribute to an increased risk of perioperative complications remains essential. These factors include patient age, comorbidities, and physical conditions. The level of anesthesia and readiness for removal of airway devices should be evaluated carefully for each patient, and quick identification of airway problems and intervention is required if patients fail to maintain the airway and sufficient ventilation after removal of airway devices.
Tracheal extubation is associated with a significant risk of complications in both adults and children. Loss of airway control in a patient after the removal of endotracheal tubes or airway devices presents a serious challenge for anesthesiologists. Failure to maintain an unobstructed natural airway and establish spontaneous respiration after extubation can lead to catastrophic respiratory complications, such as cardiovascular collapse or even lasting cerebral damage. According to the Fourth National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society (NAP4)
, one-third of major airway complications occur during emergence from anesthesia in operating rooms and in Postanesthesia care unit (PACU) [1]. However, tracheal extubation has been less focused on and discussed by anesthesiology experts than tracheal intubation, for which detailed guidelines have been published and repeatedly updated [2]. To date, extubation guidelines for adult patients have been published [3]; however, no specific guidelines have been published for extubation in pediatric patients.
In pediatric patients incapable of cooperating with and understanding the medical procedures being applied to them, airway devices often need to be removed while they are, to some degree, under the influence of anesthetics. There is a danger that the lingering effects of the remaining anesthetics can compromise airway patency and respiratory reflex, which increases the risk of perioperative airway complications in this age group [4]. The removal of endotracheal tubes and airway devices is customarily conducted in two ways in pediatric anesthesia: when patients are in a deep anesthesia or after regaining consciousness. According to the Anaesthesia PRactice in Children Observational Trial (APRICOT) [5], majority of anesthesiologists prefer awake extubation in children. Deep extubation is an advanced technique [3]. Some experts are skeptical of this method because removing airway devices from deeply anesthetized patients, whose protective airway reflex is impaired by anesthetics, may present a risk of aspiration. Anesthetized patients may be incapable of maintaining airway patency; therefore, potentially collapsed upper airways may increase the risk of airway obstruction. Conversely, many pediatric anesthesiologists prefer deep extubation and removal of supraglottic airway devices (SAD). [5]. Awake extubation is associated with the risk of coughing and spasmodic expiration with desaturation in children; however, the incidence of serious complications such as laryngospasm and breath-holding does not differ between the two methods [6,7].
In both methods, assessing the readiness of a patient and optimal timing for the removal of airway devices remains crucial for anesthesiologists. However, to date, clear criteria for safe airway management and/or standard protocols for extubation and removal of airway devices in the pediatric population have not been established. Anesthesiologists decide the readiness of a patient for extubation and the method most suitable in each case based on a few clinical cues and their own experiences. This review aims to illustrate issues associated with extubation and the removal of airway devices in children and offers readers some insights on points to consider before taking this important airway management step during emergence from anesthesia to reduce the risk to pediatric patients.
In adults, extubation or removal of SADs is almost always performed after patients are awakened from anesthesia, when they are able to obey commands and their protective airway reflexes and laryngeal muscle tone recover to preanesthetic levels. The main concerns of practitioners at this moment include coughing associated with discomfort and hemodynamic changes induced by airway stimulation. The latter is particularly important in adult patients who are often compromised by pre-existing cardiovascular conditions. However, these principles do not apply to children. Children awakened from anesthesia are often confused and become agitated in unfamiliar operating room environments; therefore, they can hardly understand or obey commands from strangers. Struggling with airway devices and pulling out tubes and catheters is not rare, and these behaviors may potentially cause children to harm themselves, leading to wound hemorrhage or dehiscence, an occurrence dreaded by surgeons.
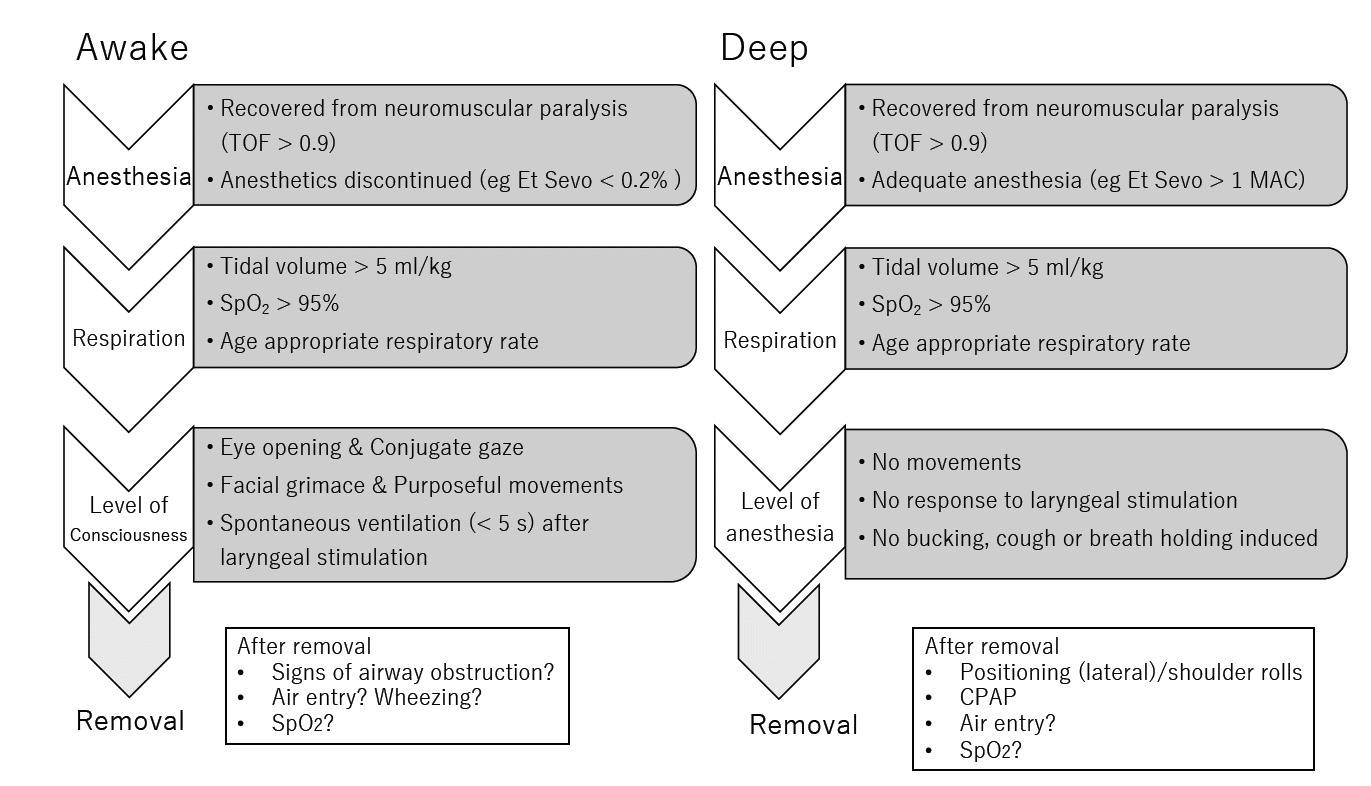
Ideally, when children emerge from anesthesia, they are calm or asleep. There should be no agitation, straining movements, kicking, or punching. After extubation or removal of airway devices, they are able to breathe without airway complications such as excessive coughing, breath-holding, panting, or airway obstruction and require the application of no more than a moderate degree of continuous positive airway pressure (CPAP) or shoulder rolls to maintain airway patency. However, achieving these clinical goals is often easier said than done, especially in young children. Children are more prone than adults to developing airway complications throughout the perioperative period because their airways are more susceptible and irritable to external stimulation [4]. Additionally, young children have naturally softer and more collapsible pharyngeal airways. Studies have demonstrated that anesthetics significantly influence airway and pharyngeal functions and alter physiological responses in a dose-dependent manner. Even in patients who are at a sub-hypnotic level of anesthesia, the effects of anesthetics are evident [8]. The effects on airway response vary according to the anesthetic used and can be modulated with the use of adjuvant agents such as opioids and lidocaine. The developmental stage of the children and the maturation of the airways should also be considered [9]. To anticipate airway responses and potential complications during emergence from anesthesia (detailed later), understanding anesthetic function and its effects on upper airway physiology in children remains essentials. This will help anesthesiologists achieve better and safer recovery from anesthesia in children.
Extubation and removal of SADs are usually conducted when patients are in one of the following two conditions: when they are at a surgical level of anesthesia and do not respond to external stimuli (deep extubation) or when they become conscious and are able to perform some purposeful movements and obey commands (awake extubation). According to the APRICOT, many anesthesiologists prefer performing awake extubation in children, probably because of concerns about irritable pediatric airways and respiratory complications [5]. However, studies have presented data showing no difference in the incidence of perioperative respiratory complications between awake and deep extubation in children [6,7]. Koo et al. [10] conducted a meta-analysis of 17 randomized control trials, and showed that deep removal of endotracheal tubes and SADs does not necessarily increase the risk of airway complications, such as laryngospasm or breath-holding. Furthermore, they showed that deep removal of endotracheal tubes and SADs reduces overall airway complication risks in children, which was contradictory to previous studies [10].
In daily practice, the tasks of selecting an optimal method and timing of extubation are decided by the anesthesiologists in charge. The tasks include the clinical evaluation of patients, such as the type of surgery performed, patients' clinical conditions, and pre-existing comorbidities, as well as the anesthesiologist’s own experience and confidence in the management of children’s airways (Fig. 1). Furthermore, the decision to remove the airway devices is made based on a careful evaluation of the recovery of a patient from anesthesia. Many studies have concluded that, extubation can be safely conducted as either awake or deeply anesthetized children if the procedure is performed adequately by experienced anesthesiologists. However, experts also agree that when patients are between the two stages of anesthesia, neither deeply anesthetized nor fully awake (i.e., in the light plane of anesthesia), extubation is unsafe and can provoke airway complications, which can lead to catastrophic events in worst-case scenarios. Therefore, the difficulty with extubation in children is not actually associated with the procedure and maneuver but in the assessment of the level of anesthesia and the readiness of the patient for airway manipulation. For either method, assessing the level of anesthesia is undoubtedly the most crucial component for the safe removal of airway devices in children.
The depth of anesthesia was first defined by Guedel in 1937. He classified the levels of anesthesia into four stages: 1 (analgesia), 2 (excitement: disinhibition and hyperreaction to external stimuli), 3 (surgical level of anesthesia), and 4 (overdose: cortex and medullary depression). Diethyl ether was the only anesthetic available during Guedel’s time; therefore, his anesthesia depth chart was based on the observation of the physiological characteristics of patients under ether anesthesia. Eventually, by the 1980s, ether was replaced with other volatile anesthetics and is no longer used. However, since there is no new substitutional classification, Guedel’s classification of anesthesia depth is still referred to today by many anesthesiologists for clinical use and education of residents and trainees. Without revised indicators to assess the level of anesthesia for the next generation of anesthetics, anesthesiologists often rely on their own experience, institutional culture, and “gut feeling” in deciding the depth of anesthesia in patients and the optimal timing for airway manipulation.
Pediatric patients vary widely in age and developmental stages. Some also have various comorbidities. This diversity in patients makes it difficult to evaluate the depth of anesthesia and further complicates determining the optimal conditions and timing for the removal of airway devices in each patient. Even in healthy, non-compromised children, visual assessment may sometimes be confusing and misleading, because lightly anesthetized and awake children sometimes respond similarly to noxious stimuli. Anesthesiologists may consider children’s reflexive responses as purposeful movements and signs that they are awake and ready for removal of airway devices. Objective measures for assessing the depth of anesthesia using various anesthetics must be established.
For awake extubation, Templeton et al. [11] conducted a prospective study on young children anesthetized with sevoflurane and investigated the criteria for safe extubation. They identified five criteria: grimace, conjugate gaze, purposeful movements, eye opening, and tidal volume > 5 ml/kg to be associated with success in awake extubation, and that the success rate increases in a stepwise manner if patients meet multiple or all of these five criteria. However, consensus on the definition of a deep level of anesthesia has never been reached, and clear criteria for the deep removal of airway devices have not been established. Previous studies on deep extubation often have not specified measurements of the depth of anesthesia or documented the protocols for extubation. The lack of criteria for extubation and the definition of “deep” anesthesia has left this method in a somewhat vague status. An end-tidal concentration of > 1 or 1.5 minimal alveolar concentration (MAC) is customarily proposed as a deep and safe level of anesthesia for extubation only when using volatile anesthetics. This is because high concentrations of volatile anesthetics eliminate the respiratory reflex to laryngeal stimulation [12]. Studies have demonstrated that sevoflurane at 0.8 MAC and desflurane 7.7%, which is approximately 1 MAC with nitrous oxide, can achieve smooth extubation in children [13,14]. However, there is no clear definition of an adequate depth of anesthesia or objective criteria for awake or deep extubation when using intravenous anesthetics or when adjuvant drugs are added to volatile anesthetics. Propofol is now one of the most commonly used anesthetics in pediatric anesthesia owing to its antiemetic effects and lower incidence of emergence delirium after anesthesia. However, clear criteria are lacking for the removal of airway devices, and there are few studies on the effect of propofol on the airways [8].
The laryngeal stimulation test is an objective test that assesses the depth of anesthesia according to the response of a patient to laryngeal stimulation and is applicable to any patient prior to extubation. Templeton et al. [11] described this simple method to determine the level of anesthesia, which involves gently moving the endotracheal tube in the cephalad and caudad directions while examining the laryngeal response of the patient. Maneuvers, such as airway suctioning or deflation of endotracheal tube cuffs, also stimulate the larynx and provoke a similar response. Gently moving supraglottic devices or deflating a cuff, if any, may provide stimuli. If a patient is in stage one, namely the awakened stage of anesthesia, laryngeal stimulation induces coughs followed by a brief pause (< 5 s) of respiration and quick recovery of spontaneous respiration. In stage two, the same stimulus provokes successive coughs followed by prolonged apnea (> 5 s), which indicates that the laryngeal systems of the patient are still under the influence of anesthetics, and extubation at this stage can provoke a strong airway response with a risk of laryngospasm and breath-holding. When patients are in the deep stage of anesthesia, namely stage 3, they usually do not respond to laryngeal or oral stimulation. Furthermore, no cessation in spontaneous respiration, or only a brief pause, is observed.
Since the end of the 20th century, patient consciousness level monitors using electroencephalograms (EEGs) have been developed and become available in anesthesia and intensive care unit (ICU) practice. The bispectral index (BIS), introduced by Aspect Medical Systems, Inc. in 1994, was one of the first developed and commercially available monitors, and is probably one of the most commonly used monitors in current practice. Its value correlates well with the consciousness level of patients under both anesthesia and sedation in ICU settings. However, certain anesthetics, including ketamine, nitrous oxide, and remimazolam [15,16], render the BIS assessment unreliable. Additionally, because young children are neurologically immature, evaluating the depth of anesthesia with the BIS in this age group is different from that in the older population. Users should acknowledge that BIS values are age-dependent. Sinha and Sood investigated BIS values in children and concluded that a BIS of approximately 60 is suitable for deep removal of a laryngeal mask airway when sevoflurane is used as an anesthetic [17]. Although the BIS can distinguish between the two stages of anesthesia, i.e. maintenance and recovery, in children, Bannister et al. [18] reported that the BIS employed in infants and young children made no difference in their recovery from anesthesia. The usefulness of EEG-based monitoring for airway management during recovery from anesthesia remains inconclusive and requires further investigation.
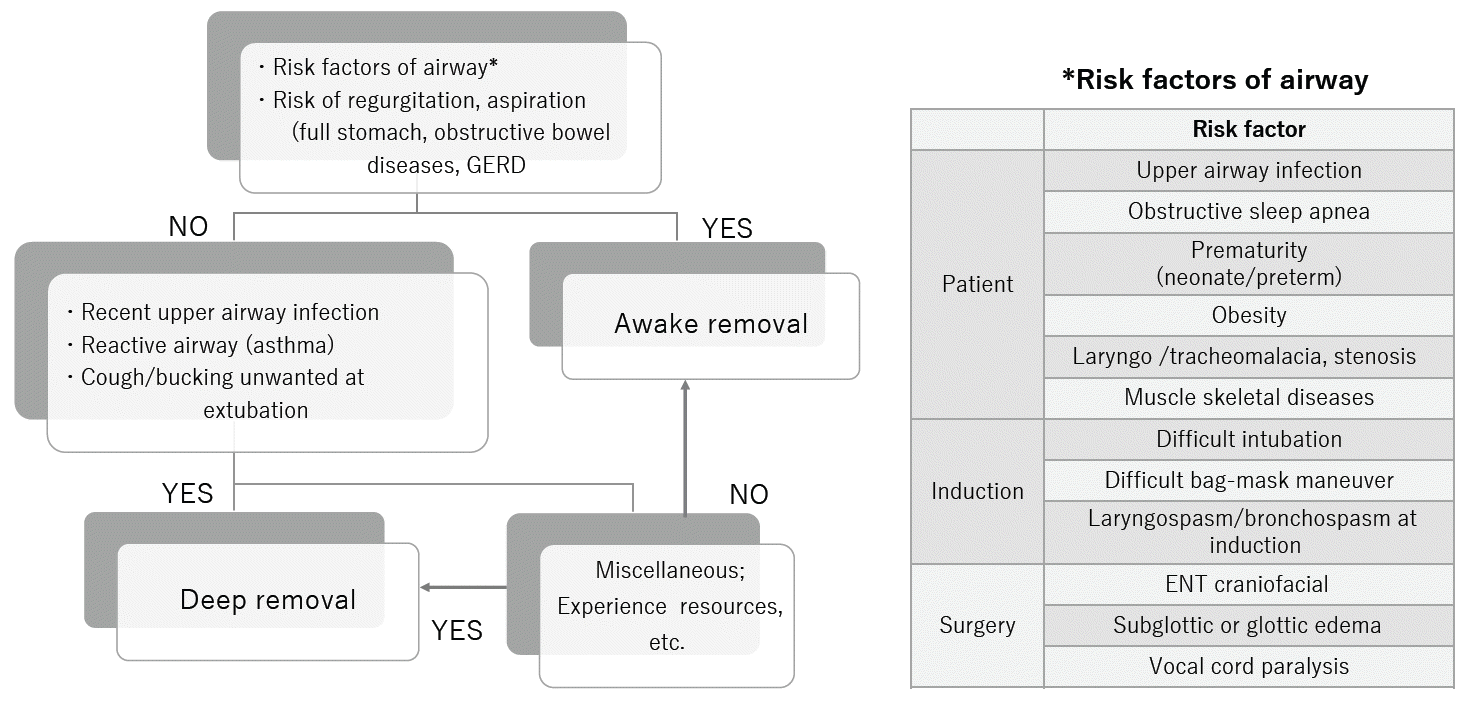
As shown by previous studies, there is no difference in the incidence of serious perioperative complications between awake and deep extubation [6,7,19]. Minor complications may occur but they rarely alter patient outcomes. Therefore, the choice of extubation method is left to the anesthesiologists, their preferences, and on-site clinical judgements [20]. How should anesthesiologists decide on the method of extubation or SAD removal? Several reviews have been published on extubation in pediatric patients and children with difficult airways [21,22]. The consensus reached by the authors was that either method is reasonably safe in patients with low-risk airways if the procedure is performed by experienced anesthesiologists. However, there are cases in which one method is generally preferred over another (Fig. 2).
Deep extubation is generally the choice for extubation in patients with reactive airways due to recent upper airway infection or asthma or after surgery in which coughing or cardiovascular changes induced by extubation are undesirable at the time of emergence from anesthesia. Patients with recent upper airway infections exhibit bronchial hyperreactivity similar to that in patients with asthma and an enhanced coughing reflex that lasts as long as four weeks after infection. Coughing increases intracranial and ocular pressure and should be avoided, especially after eye or intracranial surgery. Hypertension and tachycardia are undesirable outcomes after cranial surgery and in patients with cardiovascular conditions. The elevated cranial venous pressure induced by coughing and breath-holding also increases the risk of bleeding from the surgical site after cleft surgery and tonsillectomy. When children have a loose primary tooth, deep extubation may be suitable because biting tubes or bite blocks during emergence are common in awake extubation and may lead to dental injury and, at worst, aspiration of a dislodged tooth. Biting a reinforced endotracheal tube can lead to tube occlusion and airway obstruction, resulting in negative-pressure pulmonary edema (NPPE).
In contrast, awake extubation is recommended when difficult ventilation and/or intubation is identified before or at anesthesia induction, or when difficult reintubation is suspected after surgery and in full stomach cases. Patients with obesity and those with a history of apnea (sleep apnea, premature infants) are susceptible to the effects of the remaining anesthetics, especially opioids, which increase the risk of airway complications after the removal of airway devices. Most importantly, awake extubation is generally preferred in cases where anesthesiologists are uneasy about performing deep extubation.
The Difficult Airway Society guidelines recommend awake extubation for patients with difficult airways and urge anesthesia providers to plan possible reintubation scenarios prior to extubation [2]. If anesthesiologists decide that patients have airway risks for extubation, extubation can be delayed until patients regain full consciousness, when protective airway reflexes and mechanisms to maintain airway patency return to pre-anesthesia levels. If the concern for patient airway failure is high or if anesthesiologists are concerned about possible difficult reintubation, extubation with more resources, including the availability of experienced personnel and pediatric intensive care unit (PICU) admission, should be considered.
Although the endotracheal tube remains the gold standard for airway management, the use of laryngeal mask airways and other types of SADs is increasing in children. SADs have advantages over endotracheal tubes such as no contact with the vocal cords and no need for laryngoscopy or muscle relaxants for placement. By avoiding direct stimuli to the perilaryngeal structures, SADs have been reported to decrease the incidence of sore throat, cough, and laryngospasm in adult patients. However, because SADs are positioned above the larynx, concerns exist regarding aspiration and mispositioning of the device, especially in small children. Although recent studies favor the use of SADs in children and infants in terms of reducing perioperative respiratory complications [23], in a systematic review of children with upper airway infection, de Carvalho et al. [24] reported that laryngeal mask airway did not decrease the incidence of laryngospasm, breath-holding, and desaturation, although it significantly decreased coughing events. Koo et al. [10] conducted a meta-analysis and compared deep and awake removal of endotracheal tubes and laryngeal mask airways in children. They showed that deep removal of both the laryngeal mask airways and endotracheal tubes similarly reduced the overall airway complications, despite an increased risk of upper airway obstruction. No significant difference was observed between the laryngeal mask airway and endotracheal tube with regard to the risk of desaturation and laryngospasm, regardless of the level of anesthesia when removed. The only difference observed in their study was that while deep extubation significantly reduced the risk of cough, the level of anesthesia did not affect coughing associated with laryngeal mask airway removal [10]. Ramgolam et al. [19] conducted a survey of children with more than one respiratory risk factor and assessed the influence of the timing of laryngeal mask airway removal on the occurrence of adverse respiratory events. The incidence of laryngospasm and desaturation did not differ between early and late removal; however, late removal of the laryngeal mask was associated with more adverse events in the PACU [19]. Furthermore, awake removal may increase the risk of biting and damaging both the devices and teeth. Brain, the inventor of the original laryngeal mask airway, wrote that the laryngeal mask airway should not be removed until the patient regains consciousness and airway-protective reflexes. However, in children, deep removal of the laryngeal mask airway has some benefits and is associated with fewer adverse events than awake removal.
SADs can also be used as substitutes for endotracheal tubes or Guedel (oropharyngeal) airways in intubated patients. First introduced by Bailey in 1995 [25], this method aimed to reduce cough and cardiovascular instability in intubated patients upon emergence from anesthesia. The exchange of endotracheal tubes for SADs is conducted in patients who are deeply anesthetized; therefore, cough and cardiovascular changes associated with extubation can be avoided. The SADs are removed once the patients regain consciousness. However, this is an advanced technique and its efficacy in pediatric patients has not been verified.
What are the consequences of poor airway management during emergence from anesthesia in children? Murat et al. [26] reported that there were 724 anesthesia-related adverse events out of 24,165 total cases in a pediatric teaching hospital, and that 53% of these events were respiratory events. According to the pediatric perioperative cardiac arrest registry, 27% of cardiac arrests are due to respiratory causes and 6% of all cases are caused by laryngospasm. Of the 11 cardiac arrest cases in the registry, seven developed laryngospasm during and after emergence from anesthesia [27].
The Frank Bird safety pyramid states that for every serious incident, there may be 30 similar damage-causing and 600 near-miss incidents. Therefore, it is natural to conjecture that behind a serious adverse event at emergence from anesthesia, there may be multiple “near-miss” events. In a report from Boston Children’s Hospital, 193 anesthesia-related patients called for help during perioperative events between 2011 and 2015. Among them, 144 calls were for respiratory causes, and 85% of the calls were made during emergence and when patients were in PACU. Desaturation and laryngospasm were the most frequent respiratory-related reasons for the calls [28]. In this report, children’s age, history of prematurity, American Society of Anesthesiologists physical status of 3–5, and coexisting respiratory illness were identified as risk factors for such incidents. These studies suggest that anesthesia-related adverse events during emergence from anesthesia, which may be minor to severe, occur more frequently than previously thought. Many of these are due to respiratory causes, and laryngospasm is a major cause of adverse events, possibly leading to severe consequences.
The incidence of laryngospasm in children is 17.4/1000. Children are at a higher risk of developing laryngospasms than adults. Upon emergence from anesthesia, movement or manipulation of the airway, suctioning, removal of airway devices, and even turning the head of a patient to the side can cause potent laryngeal stimuli and provoke an exaggerated response in the larynx, leading to partial or complete closure of the vocal cords and airway obstruction. The risk factors for laryngospasm in children include younger age, recent upper airway infection, asthma, exposure to cigarette smoke, and surgical procedures involving the airway and pharynx, such as tonsillectomy and adenoidectomy. The use of desflurane, an airway irritant and volatile anesthetic, is considered to increase the risk of laryngospasm; however, Cranfield and Bromley [14] reported that extubation may be successfully managed with a high concentration of desflurane. Volatile anesthetics at low concentrations (< 1 MAC) increase the risk of laryngospasm. With an insufficient depth of anesthesia, stimulation by foreign objects such as endotracheal tubes, secretions, or blood pooled in the pharynx touching the periglottis structures or advancing into the trachea could be potent triggers inducing protective airway reflexes. In conscious humans, the laryngeal reflex is under inhibitory control from the higher cortex, and glottic closure does not last for a long period. Therefore, airway manipulation in conscious patients is considered less likely to cause prolonged laryngospasm. An animal study also showed that hypercapnia and hypoxia (PaO2 < 50 mmHg) have depressant effects on this reflex. This phenomenon may be a last resort for anesthesiologists who rely on the spontaneous resolution of laryngospasm under severe hypoxia; however, this is not a generally accepted approach for laryngospasm management in practice.
Perioperative laryngospasm occurs most frequently during induction and emergence from anesthesia. For prevention, airway manipulation, especially extubation and removal of SADs, should be performed while patients are in a deep plane of anesthesia or awake. Neither technique is superior for laryngospasm prevention. In the “No touch” extubation technique for prevention of laryngospasm in children, Tsui et al. [29] emphasized the importance of avoiding stimulation while patients were in a light plane of anesthesia, and recommended extubating patients after they had spontaneously opened their eyes. Studies have shown that the administration of magnesium and lidocaine before extubation may help prevent laryngospasms, although the effect of these agents is limited [4,30].
Bronchospasm potentially arises at any stage of anesthesia, more often at induction but also during emergence from anesthesia. Mechanical pharyngeal stimulation associated with the movement of tracheal tubes or secretions and suctioning of the airways can trigger this sudden airway response. The use of SADs and deep extubation has been advocated in the literature as measures to reduce the risk of bronchospasm. However, aspiration with a dislodged laryngeal mask airway can cause perioperative bronchospasm, and a study of 831 children by von Ungern-Sternberg suggested that the use of the device is not a guaranteed solution for this complication if the children are at risk [31]. Children with asthma or a history of acute or recent upper airway infection (URI) are at a risk of bronchospasm because their airways have already become hyperreactive and respond to external airway stimuli. Children whose caretakers are habitual cigarette smokers are also at risk. Even after uneventful extubation, bronchoconstriction can be triggered, which can result in serious consequences for the vulnerable, especially young children. Therefore, prophylaxis for optimizing preoperative conditions, such as taking asthma medications, including steroids, and perioperative parental abstinence from cigarettes, is important. Additionally, cancellation of an operation in cases of acute URI should be discussed with surgeons. Due to its parasympathomimetic effect, neostigmine can trigger bronchospasm even in patients without a history of allergies or asthma; therefore, it should be used with caution. The use of sugammadex has recently been advocated for the reversal of rocuronium and vecuronium and is useful for the reversal of muscle relaxation in patients at risk of hyperreactive airways because it has different mechanisms of action for the reversal of muscle relaxants and may reduce respiratory complications after anesthesia.
Upper airway obstruction is commonly observed during emergence from anesthesia in both adults and children, particularly after deep extubation. Among the muscles in the human body, pharyngeal muscles are the most sensitive to the residual effects of neuromuscular blocking agents (NMBA) and anesthetics. Furthermore, various anesthetics, including volatile anesthetics, propofol, and sedatives such as dexmedetomidine, depress the inspiratory activity of the pharyngeal muscles. The diaphragm and intercostal muscles are less sensitive and recover faster from the effects of NMBA and anesthetics. Because of the difference in the sensitivity and speed of recovery of these respiratory muscles, collapse of the upper airway often develops after removal of airway devices as a result of the imbalance in muscle tone. This is caused by failure of insufficient pharyngeal muscle strength to counteract the negative pressure generated by the inspiratory force of the diaphragm and intercostal muscles. Therefore, it is not surprising that deep extubation is more associated with upper airway obstruction than awake extubation [6]. With either method of extubation, complete reversal of NMBA is essential, and the role of the pharyngeal muscle in maintaining pharyngeal space and upper airway patency is crucial once airway devices are removed and patients are left with their “natural” airways. The risk of upper airway obstruction is high in patients with obstructive sleep apnea syndrome (OSAS) with hypertrophic tonsils, microretrognathia, macroglossia, obesity, or laryngomalacia. Children with Down syndrome have multiple preoperative risk factors, and are prone to upper airway obstruction. Surgery, such as cleft palate repair, which potentially induces postoperative lingual swelling, is also a risk factor. For prevention and treatment, maneuvers such as placing shoulder rolls, placing patients in a lateral position to reduce the gravitational impact on oral structures (soft palate, tongue, and tonsils), or simply positioning the head of the patient sideways are necessary. Moderate CPAP using a mask is effective in maintaining upper airway patency, especially in neonates and infants. In cases of micrognathia or OSAS with hypertrophic tonsils, the insertion of a nasopharyngeal airway before extubation may be helpful. In patients with laryngomalacia, CPAP or high-flow nasal cannula (HFNC) immediately after extubation is often helpful. HFNC provides an aero-splinting effect, helps maintain upper airway patency, and minimizes efforts for breathing and inspiration in patients.
Postextubation NPPE is a relatively rare occurrence among children. Tsai et al. [32] investigated 8,556 anesthesia records of patients and reported that the incidence of postextubation NPPE was 0.019%. All patients with NPPE were adults, and their risk factors were smoking and endotracheal intubation. NPPE develops after extubation or removal of airway devices, and obstruction after laryngospasm is the major cause of NPPE in adults. However, cases of NPPE during emergence from anesthesia have been documented in older children. NPPE after airway obstruction is caused by forced inspiratory efforts against a closed glottis or an obstructed airway, which generates intense negative intrathoracic pressure and draws capillary and interstitial fluids into the alveoli, leading to edema. Onset is usually acute; however, symptoms resolve quickly in response to supportive therapies, such as positive end-expiratory pressure (PEEP).
Warner et al. [33] reported that perioperative aspiration occurred once in every 2,632 infants and children. In their cohort, three of 24 patients who experienced pulmonary aspiration exhibited clinically significant symptoms and required respiratory support; however, no mortality was reported. Most reported cases of aspiration occurred during induction. Emergency cases without preoperative fasting and patients with bowel obstruction diseases were more likely to experience regurgitation and vomiting. In this study, the authors did not specify the use of anesthetics or the method of extubation; however, they mentioned that in their institution, infants and young children generally underwent awake extubation. While there are no studies investigating the incidence of pulmonary aspiration in children who were extubated under deep anesthesia, nor a comparison of the incidence between the two methods, PICU patient studies have suggested that assessing gastric volume before extubation using point-of-care ultrasonography and placing patients in the lateral position before extubation may help reduce the risk of regurgitation and aspiration [34]. However, in children with a full stomach, obstructive bowel disease, oropharyngeal dysfunction, or gastroesophageal reflux disease, extubation under deep anesthesia may further increase the risk of pulmonary complications; therefore, awake extubation is recommended.
Children often exhibit signs of distress, pain, and discomfort as well as negative behaviors such as emergence delirium and agitation during the recovery phase from anesthesia. Children are apt to develop emergence delirium, especially after the cessation of volatile anesthetic administration. Studies have shown conflicting results regarding whether extubation in deeply anesthetized children helps decrease agitated behavior during emergence from anesthesia [35,36]. Various adjuvant agents, such as propofol, opioids, lidocaine, and magnesium sulfate, have been combined with volatile anesthetics to investigate whether these agents can reduce the agitated behavior of children during emergence from anesthesia. While the prophylactic effects of these agents remain inconclusive, α-2 agonists clonidine and dexmedetomidine have been investigated for their effects on emergence delirium and shown to be effective without delaying the time of PACU discharge. Dexmedetomidine has hypnotic and analgesic properties and can reduce the required dose of sevoflurane and isoflurane intraoperatively and help promote smooth awake extubation in children.
The application of positive airway pressure as a tracheal tube is removed aims to reduce atelectasis and improve postoperative oxygenation. This maneuver is commonly performed by anesthetists or ICU practitioners in adult patients. Some researchers believe that the applied pressure and increased airflow may have an additional effect of blowing off accumulated secretions above the pharyngeal structures and preventing these materials from entering the trachea, thereby lowering the risk of laryngospasm and other pulmonary complications. However, there is no robust evidence supporting this theory. One study reported that the onset of desaturation after tracheal tube removal is delayed in children in whom positive pressure is applied with 100% oxygen prior to extubation, compared to children who are extubated in a conventional way and exposed to negative airway pressure before extubation with suctioning [37]. The application of negative pressure prior to extubation with excessive airway suctioning is not recommended in practice. The lung recruitment maneuver, which reopens the atelectatic alveoli before extubation, is beneficial in small children with limited oxygen reserves due to low functional residual capacity. However, the timing of applying positive airway pressure in spontaneously breathing patients, manner of pressure application (continuous or one-time inflation), and optimal pressure in children require further investigation.
When patients cannot sustain adequate spontaneous respiration, signs of respiratory distress (dyspnea or tachypnea, wheezing, severe airway obstruction, or desaturation) and extubation failure should be suspected. Rapid identification of the causes of respiratory problems and appropriate interventions are required. Reintubation to re-establish the airway of the patient is also considered. Reportedly, the incidence of perioperative reintubation after planned extubation in children is 0.0026–0.0096%, which is much lower than the incidence in PICU patients. However, 22% of perioperative reintubation cases were found to be associated with serious adverse events, such as resuscitation attempts including chest compressions, which resulted in prolonged intubation and admission to PICU [38]. The causes of reintubation were mostly respiratory in this cohort, and the average age of the reintubated patients was 3.7 ± 4.8 years, compared to 7.0 ± 5.9 years of overall patients. Murat et al. [26] reported that infants under one year of age had a higher rate of reintubation than older children.
Difficult reintubation can occur when patients are at risk prior to surgery or when surgery induces anatomical and functional changes in the airway. Although extubation and removal of airway devices is always an elective procedure, and the timing of extubation is subjective depending on each anesthesiologist’s “game plan,” reintubation is often not predicted and back-up plans for extubation failure may not exist. In patients who have lost airway function and are rapidly deteriorating in ill-prepared conditions with inadequate resources, reintubation can sometimes be extremely difficult. Equipment, facilities (for extubation in the operation room or PICU), and human resources (skilled individuals for assistance) should always be available or in close proximity at the time of extubation. Jagannathan et al. [39] reported that of 137 children with difficult airways, 130 had successful extubation; however, seven failed, including one who underwent tracheostomy and two who had cardiac arrest. The risk-benefit evaluation of awake extubation versus extubation before the return of consciousness should be thoroughly considered before extubation in patients at risk of airway reintubation. Awake extubation is strongly recommended in patients with difficult airways.
Although proven safe and recommended in adult cases, experience in using airway exchange catheters in pediatric patients is limited, and the 2022 American Society of Anesthesiologists guidelines recommend minimizing their use in children [2]. However, a clinical investigation by Wise-Faberowski and Nargozian [40] demonstrated the use of airway exchange catheters in 20 PICU children with difficult airways and 5 patients in whom the catheter was actually used for reintubation. Although safety validation in the pediatric population is still needed, the use of an airway exchange catheter is worth considering for extubation in cases of difficult airways.
Coughing and airway manipulation generate airborne particles and droplets, spread pathogens, and increase the chances of transmission of infectious diseases to healthcare providers. Extubation generates more aerosol particles than intubation, particularly when extubation is associated with coughing. The risk of occupational health hazards has been highlighted during the recent coronavirus pandemic, making anesthesiologists aware of the health risks to themselves and colleagues posed by their routine airway practices. Anesthesiologists must prioritize the prevention of the spread of airborne diseases among healthcare providers and keep the medical environment safe for workers. Reducing the spread of pathogens should always be considered when managing airways in perioperative and ICU settings.
The removal of airway devices is a crucial step in recovery from anesthesia. It is often associated with adverse respiratory events. In children, whose oxygen reserve is low and airway response is different from that in adults, it sometimes leads to severe complications. Careful assessment of the readiness of a patient and their ability to maintain an unobstructed natural airway is mandatory prior to the removal of airway devices. Maneuvers and resources to manage airway complications during emergence from anesthesia or extubation failure need to be considered as part of perioperative airway management.
REFERENCES
1. Cook TM, Woodall N, Frerk C; Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011; 106:617–31.

2. Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology. 2022; 136:31–81.

3. Difficult Airway Society Extubation Guidelines Group, Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia. 2012; 67:318–40.

4. Erb TO, Trachsel D, von Ungern-Sternberg BS. Laryngeal reflex responses in pediatric anesthesia. Pediatr Anesth. 2020; 30:353–61.

5. Engelhardt T, Virag K, Veyckemans F, Habre W; APRICOT Group of the European Society of Anaesthesiology Clinical Trial Network. Airway management in paediatric anaesthesia in Europe-insights from APRICOT (Anaesthesia Practice In Children Observational Trial): a prospective multicentre observational study in 261 hospitals in Europe. Br J Anaesth. 2018; 121:66–75.

6. Baijal RG, Bidani SA, Minard CG, Watcha MF. Perioperative respiratory complications following awake and deep extubation in children undergoing adenotonsillectomy. Paediatr Anaesth. 2015; 25:392–9.

7. Von Ungern-Sternberg BS, Davies K, Hegarty M, Erb TO, Habre W. The effect of deep vs. awake extubation on respiratory complications in high-risk children undergoing adenotonsillectomy: a randomised controlled trial. Eur J Anaesthesiol. 2013; 30:529–36.
8. Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005; 103:470–7.

9. Isono S. Developmental changes of pharyngeal airway patency: implications for pediatric anesthesia. Paediatr Anaesth. 2006; 16:109–22.

10. Koo CH, Lee SY, Chung SH, Ryu JH. Deep vs. awake extubation and LMA removal in terms of airway complications in pediatric patients undergoing anesthesia: a systemic review and meta-analysis. J Clin Med. 2018; 7:353.

11. Templeton TW, Goenaga-Díaz EJ, Downard MG, McLouth CJ, Smith TE, Templeton LB, et al. Assessment of common criteria for awake extubation in infants and young children. Anesthesiology. 2019; 131:801–8.

12. Erb TO, Von Ungern-Sternberg BS, Moll J, Frei FJ. Impact of high concentrations of sevoflurane on laryngeal reflex responses. Paediatr Anaesth. 2017; 27:282–9.
13. Inomata S, Suwa T, Toyooka H, Suto Y. End-tidal sevoflurane concentration for tracheal extubation and skin incision in children. Anesth Analg. 1998; 87:1263–7.

14. Cranfield KA, Bromley LM. Minimum alveolar concentration of desflurane for tracheal extubation in deeply anaesthetized, unpremedicated children. Br J Anaesth. 1997; 78:370–1.
15. Mishra RK, Mahajan C, Prabhakar H, Kapoor I, Bithal PK. Effect of nitrous oxide on bispectral index values at equi-minimum alveolar concentrations of sevoflurane and desflurane. Indian J Anaesth. 2017; 61:482–5.
16. Shirozu K, Nobukuni K, Tsumura S, Imura K, Nakashima K, Takamori S, et al. Neurological sedative indicators during general anesthesia with remimazolam. J Anesth. 2022; 36:194–200.
17. Sinha A, Sood J. Safe removal of LMA in children - at what BIS? Paediatr Anaesth. 2006; 16:1144–7.
18. Bannister CF, Brosius KK, Sigl JC, Meyer BJ, Sebel PS. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001; 92:877–81.
19. Ramgolam A, Hall GL, Zhang G, Hegarty M, Von Ungern-Sternberg BS. Deep or awake removal of laryngeal mask airway in children at risk of respiratory adverse events undergoing tonsillectomy-a randomised controlled trial. Br J Anaesth. 2018; 120:571–80.

20. Templeton TW, Sommerfield D, Hii J, Sommerfield A, Matava CT, Von Urgen-Sternberg BS. Risk assessment and optimization strategies to reduce perioperative respiratory adverse events in Pediatric Anesthesia-Part2: Anesthesia-related risk and treatment options. Paediatr Anaesth. 2022; 32:217–27.
22. Weatherall AD, Burton RD, Cooper MG, Humphreys SR. Developing an Extubation strategy for the difficult pediatric airway-Who, when, why, where, and how? Paediatr Anaesth. 2022; 32:592–9.

23. Drake-Brockman TF, Ramgolam A, Zhang G, Hall GL, Von Ungern-Sternberg BS. The effect of endotracheal tubes versus laryngeal mask airways on perioperative respiratory adverse events in infants: a randomised controlled trial. Lancet. 2017; 389:701–8.

24. De Carvalho ALR, Vital RB, De Lira CCS, Magro IB, Sato PTS, Lima LHN, et al. Laryngeal mask airway versus other airway devices for anesthesia in children with an upper respiratory tract infection: a systematic review and meta-analysis of respiratory complications. Anesth Analg. 2018; 127:941–50.

25. Nair I, Bailey PM. Use of the laryngeal mask for airway maintenance following tracheal extubation. Anaesthesia. 1995; 50:174–5.
26. Murat I, Constant I, Maud'huy H. Perioperative anaesthetic morbidity in children: a database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth. 2004; 14:158–66.

27. Bhananker SM, Ramamoorthy C, Geiduschek JM, Posner KL, Domino KB, Haberkern CM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg. 2007; 105:344–50.

28. Vlassakova BG, Sinnott SM, Askins N, Callahan MX, Leahy IC, Zurakowski D, et al. The anesthesia perioperative "call for help"-experience at a quaternary pediatric medical center: analysis of 67,564 anesthesia encounters. Anesth Analg. 2018; 127:126–33.

29. Tsui BCH, Wagner A, Cave D, Elliott C, El-Hakim H, Malherbe S. The incidence of laryngospasm with a "no touch" extubation technique after tonsillectomy and adenoidectomy. Anesth Analg. 2004; 98:327–9.

30. Gulhas N, Durmus M, Demirbilek S, Togal T, Ozturk E. Ersoy MO. The use of magnesium to prevent laryngospasm after tonsillectomy and adenoidectomy: a preliminary study. Pediatric Anesth. 2003; 13:43–7.
31. Von Ungern-Sternberg BS, Boda K, Schwab C, Sims C, Johnson C, Habre W. Laryngeal mask airway is associated with an increased incidence of adverse respiratory events in children with recent upper respiratory tract infections. Anesthesiology. 2007; 107:714–9.

32. Tsai PH, Wang JH, Huang SC, Lin YK, Lam CH. Characterizing post-extubation negative pressure pulmonary edema in the operating room—a retrospective matched case-control study. Perioper Med (Lond). 2018; 7:28.

33. Warner MA, Warner ME, Warner DO, Warner LO, Warner EJ. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999; 90:66–71.

34. Nabialek T, Tume LN, Cercueil E, Morice C, Bouvet L, Baudin F, et al. Planned peri-extubation fasting in critically Ill children: an international survey of practice. Front Pediatr. 2022; 10:905058.

35. Lee YC, Kim JM, Ko HB, Lee SR. Use of laryngeal mask airway and its removal in a deeply anaesthetized state reduces emergence agitation after sevoflurane anaesthesia in children. J Int Med Res. 2011; 39:2385–92.

36. Valley RD, Freid EB, Bailey AG, Kopp VJ, Georges LS, Fletcher J, Keifer A. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of desflurane and sevoflurane. Anesth Analg. 2003; 96:1320–4.

37. Guglielminotti J, Constant I, Murat I. Evaluation of routine tracheal extubation in children: inflating or suctioning technique? Br J Anaesth. 1998; 81:692–5.

38. Ing C, Chui I, Ohkawa S, Kakavouli A, Sun L. Incidence and causes of perioperative endotracheal reintubation in children: a review of 28,208 anesthetics. Paediatr Anaesth. 2013; 23:621–6.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download