Abstract
Pediatric regional anesthesia (RA) has emerged as a rapidly advancing dimension within pediatric anesthesia, demanding a continual commitment to knowledge acquisition. This review underscores the contemporary significance of this specialty, focusing on its application in neonates and infants. The primary objective of RA is to address perioperative pain effectively while preserving the delicate physiological balance, thereby enhancing overall patient care. This review explores the advantages offered by RA in this age group. Furthermore, conventional, and recently introduced techniques of RA are examined by exploring the advantages and disadvantages of these methods. The aim is to provide clinicians with a nuanced understanding of their applicability in different clinical scenarios. Additionally, the review elucidates the unique considerations associated with pediatric RA, acknowledging pediatric patients’ distinctive anatomical and physiological characteristics. The exceptional cases of congenital anomalies and their implications for the choice of RA are considered. An aspect of the review is its focus on dosages of local anesthetics and the volumes required for various blocks in neonates and infants. The dosages for continuous infusion and practical issues with infusions are considered. Complications associated with RA are described, along with their prevention and treatment. The review offers pragmatic insights into the selection criteria for various regional blocks, aiding anesthesiologists in making informed decisions tailored to individual patient needs.
Unlike opioids, regional anesthesia (RA) plays a crucial role in pediatric anesthesia by providing analgesia without respiratory complications and nausea. In addition to pain reduction, RA diminishes the autonomic, humoral, metabolic, immunologic, and neuroendocrine stress response to surgery. Neuraxial block improves gastrointestinal peristalsis through its vasodilatory effect on splanchnic circulation [1]. This review cannot consider the detailed description of each RA technique in neonates and infants, however, it does present the essence of what RA offers to this most vulnerable population.
The potential risks associated with general anesthesia (GA) in neonates and infants, and the benefits of RA in mitigating these risks and promoting better postoperative outcomes, should be recognized. The risks associated with GA include:
Higher incidence of hypoxia, bradycardia, and postoperative apnea: the incidence of these events is higher in neonates and infants than in older children. RA limits the need for GA and opioids, hence it may reduce these events and produce better outcomes [2].
Susceptibility to prolonged effect of general anesthetics: immature renal and hepatic systems delay drug metabolism, resulting in protracted effects of anesthetic drugs [3].
Effect on neurodevelopmental outcomes: although not unequivocally proven, general anesthetics may affect the developing nervous system. Furthermore, prolonged use of opioids and benzodiazepines was found to be associated with a risk of poorer neurodevelopmental outcomes [4].
The use of RA reduces the need for inhalational anesthetics and opioids, leading to faster wake-up times, smoother emergence, and earlier discharge.
The infant’s safety is the top priority, and constant vigilance, along with appropriate monitoring, helps ensure a smooth and secure administration of RA in this vulnerable population.
Monitoring, such as pulse oximetry, electrocardiography, and capnography, is essential. When placing patients in the lateral position for specific blocks, one must be mindful of the face mask, laryngeal mask airway, or endotracheal tube, as disconnections can occur. Safety measures include selecting a blockade that aligns with the surgery’s specific nature and the anesthesiologist’s expertise. Meticulous dose and volume calculations are crucial. The local anesthetic (LA) should be injected with repeated aspiration and in aliquots [5]. Each aliquot should be treated like a test dose. Vigilance and awareness of overall inputs, such as respiration (if on spontaneous respiration), the sound of the pulse oximeter, and alarms, are skills that need honing.
With appropriate expertise and specific indications, many regional techniques can be performed. This section will address the procedures and blocks most frequently performed in this age group.
All RA modalities can be applied to this versatile and time-tested block. We will discuss the landmark, peripheral nerve stimulator (PNS), and ultrasound (US) guided techniques. Aseptic precautions are standard, regardless of the modality.
Position of the patient: lateral decubitus position, gentle flexion at the hip and knee (Fig. 1A).
Procedure: The sacral hiatus is identified by palpating bony prominences known as sacral cornua (Fig. 1B) [6]. A hypodermic needle (24 to 22 G) is inserted at a 30–45° angle until a loss of resistance (LOR) is felt, indicating penetration of the sacrococcygeal membrane. LA is incrementally injected using repeated aspiration to detect any blood or cerebrospinal fluid (CSF).
The position of the patient and the procedure is like the landmark technique, except that a 22 G, 50 mm insulated needle is used to penetrate the sacrococcygeal ligament. The needle is inserted by an anesthesiologist as in the landmark approach. Once the space is confirmed, another anesthesiologist (assistant) connects the nerve stimulator to the 22 G insulated needle and applies the neurostimulation at 3–5 mA current. If anal sphincter contraction (innervation from S2 to S4) is elicited at 3 mA, it is considered as an endpoint. LA is injected after confirming negative aspiration for blood, CSF, and subcutaneous bulging.
Table 6 illustrates the possible positions of the needle followed by inference, implications, and troubleshooting.
In a similar position, the 7–13 MHz linear high-frequency transducer is first placed transversely to get the hyperechoic sacral cornua in transverse view along with the hypoechoic sacral hiatus and two band-like hyperechoic structures (Fig. 2A, B). The sacrococcygeal ligament and dorsal surface of the sacral bone are identified. A longitudinal view of the sacral hiatus is taken by rotating the transducer 90°. The hypodermic needle (22–24 G) is inserted using the in-plane approach (Fig. 3A, B). Needle entry into the caudal canal, which is identified as a hypoechoic canal tapering off caudally and bordered by dorsal and ventral hyperechoic bands, is visualized in real-time. The LA is injected after negative aspiration. The spread of LA is seen as tissue displacement within the sacral canal. The probe can be moved cephalad, horizontally or transversely, to see the “dural sag” (Fig. 3C), a surrogate marker for drug deposition.
The dosage of 0.25% bupivacaine is 0.5, 1, and 1.25 ml/kg for sacral, lumbar, and thoracic dermatomes, respectively.
A continuous catheter may also be introduced in the caudal space through the caudal epidural port, and it can be threaded up to thoracic levels to get congruent segment analgesia. Valairucha et al. [7] found no difference in the incidence of complications between the direct lumbar/thoracic versus caudal approaches. However, they found that 28 out of 86 (32.6%) caudally placed thoracic catheters needed to be more adequately positioned when confirmed radiographically. In this regard, evaluating that the catheter tip is appropriately positioned in the required dermatome when a thoracic or lumbar epidural catheter is placed indirectly through the caudal route is more prudent.
It has also been shown that the use of US improves the accuracy of placement of catheters at the surgically congruent vertebral level, especially in neonates and infants, the age group in which the central neuraxial conduit is best visualized (Fig. 4A) [8]. Fig. 4B shows the transverse scan at the thoracic level and the RA possibilities around it.
Recently, the author has explored the nuances of caudal epidural in preterm neonates and has concluded that when US is not available, needle insertion less than 3 mm/kg beyond the puncture of the sacrococcygeal membrane should prevent dural contact in 99.9% of neonates [9]. Common issues with continuous caudal epidural catheters in neonates and infants are given in Table 7.
Lumbar and thoracic site-specific continuous epidurals can be placed in neonates and infants. The expertise and training of the team are essential for the management of infusions and troubleshooting. The equipment must be customized. The preferred needle for neonates and infants is a 20 G, 5 cm Tuohy needle with a 22 G catheter, or 19 G needle with a 21 G catheter.
Exploratory laparotomies in infants and open thoracotomies are the indications encountered in day-to-day practice. We have also uncovered the impact of continuous thoracic epidural in controlling arrhythmias. This discovery reduces the antiarrhythmic agent load and validates the effectiveness of the irreversible destination therapy - surgical thoracic sympathectomy [10]. Detecting LOR with saline is preferred. However, air is also used by some authors to detect the epidural space. Alternately, nerve stimulation guidance or US guidance is used.
Caveat: This technique is reserved for the seasoned pediatric anesthesiologist. The potential complications are severe, such as spinal cord injury, paraplegia, total spinal and LA, and anesthesia systemic toxicity (LAST). Commonly used drugs and dosages are provided in Table 8.
Sick neonates or preterm babies are most benefitted by SA, as it can be a sole anesthetic [11]. Since it can be administered when the patient is awake, the risk of postoperative apnea associated with anesthetic drugs is nullified. The cardiorespiratory disturbances due to SA are also minimal [12]. In neonates, the dural sac ends at S3 and the spinal cord at L3 vertebral level. The Tuffier’s line passes through L4–5/L5–S1 and forms the landmark for needle introduction. Hyperbaric or isobaric bupivacaine at a dose of 0.5 mg/kg is the most common choice [13]. Spinal blockade in infants, compared to adults, has a shorter duration of analgesia, less dense blockade, minimal failure rate, and a lower risk of high spinal block requiring resuscitation.
The indications and scope of upper extremity blocks in infants and neonates are unique. Unlike older children, these cases are primarily associated with infective conditions, such as osteomyelitis of the joints, or congenital anomalies like radial club hand repair and syndactyly. Trauma to the upper limb is rare in this age group, with supracondylar fractures and finger injuries being more common than shoulder and humerus traumas.
The most common blocks are supraclavicular, infraclavicular and axillary approaches to the brachial plexus block, although the interscalene approach can also be used.
The supraclavicular approach encompasses all surgeries involving the humerus and below. Historically, this block fell out of favor before the widespread use of US due to the potential risk of damaging the pleural dome in the root of the neck [14]. However, with real-time US guidance, the block has regained popularity as the US guidance allows for the avoidance of such damage [15]. Nevertheless, complications, including arterial and pleural puncture, are recognized risks associated with this frequently utilized block.
In infants and neonates, the infraclavicular block has emerged as a valuable option. Various methods for performing an infraclavicular block have been outlined; we advocate a lateral approach for needle insertion, either below or next to the coracoid process. This technique reduces the risk of pneumothorax, particularly when compared to more medially positioned injection sites closer to the pleura [16]. The lateral coracoid approach indicates that the needle entry point is not below the midpoint of the clavicle but is guided by the coracoid process. It is useful in surgeries involving the distal humerus, elbow, forearm, and hand. This block covers the nerves spared in the axillary approach, specifically the musculocutaneous and axillary nerves [17]. It is well-suited for catheter placements due to its anatomy. However, caution is warranted, as vascular punctures and pneumothorax are potential complications [18]. Neonates and infants differ from adults, in that the practice of injecting LA at the posterior cord is highly recommended in adults [19]. However, the injection at either the lateral or posterior cord is equally effective in neonates and infants because the fibrous tissues, connective tissues, and sheaths around the neuro-vasculature are supple [20]. This suppleness makes them more amenable to the action of LA, allowing the LA to percolate better compared to adult patients. Furthermore, it reduces unnecessary needle passes in an attempt to reach the posterior cord, especially considering its proximity to the pleura.
We have discovered that injection at the lateral cord is equally effective (Fig. 5A, B) [20]. The insertion of the infraclavicular catheter in infants is well-documented in the literature and has proven beneficial, particularly in invasive surgeries that cause intense postoperative pain [20]. The most encountered indication we face is radial clubhand repair. The use of US is particularly advantageous in this scenario as the radius is absent, muscles are not developed, and the end motor response is ambiguous [21].
The axillary approach is primarily recommended for wrist and finger surgeries, with arterial damage being a recognized complication associated with this approach.
While this approach is uncommon in neonates and infants, the author has nonetheless employed this approach in specific, carefully chosen cases. One such instance involved a neonate with shoulder joint osteomyelitis, nearing septicemia [22]. This strategic utilization helped circumvent the need for GA, preserving the delicate physiological milieu.
In neonates and infants, the caudal epidural portal is easily accessible, and one may not really find it mandatory to hone skills in these blocks. However, lower limb blocks should not be overlooked; they are a crucial option because the evidence supports the notion that peripheral nerve blocks provide prolonged analgesia compared to US-guided caudal blocks [23]. Furthermore, extended analgesia, sparing the contralateral limb, and preventing urinary retention enhance the specificity of RA.
Adequate anesthesia in unilateral lower limb surgeries can be achieved by combining femoral (Fig. 6A) and sciatic nerve (Fig. 6B) blocks [24], or by using the fascia iliaca compartment block (FICB). These blocks can be administered with the precision of US and PNS guidance. FICB, performed under US guidance, is ideal for anterolateral thigh surgeries. The femoral block ensures analgesia for the anterior thigh and femur, while subgluteal, intragluteal, and popliteal sciatic nerve blocks prove effective for surgeries below the knee [25,26].
Common indications in infants include club foot repair and quadriceps plasty, particularly in arthrogryposis. Lower limb trauma is rare in our practice within this age group. Congenital anomalies, while thought-provoking, pose challenges, even with the precision of US guidance. Arthrogryposis complicates sciatic nerve identification due to the similar appearance of tendons and nerves in the traverse section, given the muscle composition dominated by fibrous tissues. PNS is also challenging due to fused or deformed joints, resulting in ambiguous motor responses [27]. This complexity emphasizes the significance of considering the central neural axis. The conclusion emphasizes the necessity for astute case-by-case assessment and careful selection of RA, akin to any other age group. However, a dual-modality approach may prove beneficial. If the sonoanatomy of the nerve is unequivocally clear, and a concurrent end motor response cannot be elicited by PNS, the author opts to administer the LA regardless of the motor response [28].
As described by the author, continuous sciatic and femoral blocks are also an option with simple equipment and techniques [29]. However, postoperative care must be diligently administered. The choice between continuous or single-shot blocks aligns with the invasiveness of the surgery.
US has enabled multiple fascial plane blocks. These include posterior rectus sheath block, ilioinguinal and iliohypogastric nerve block, transversus abdominis plane (TAP) block, subcostal TAP block, quadratus lumborum block, erector spinae block, and chest wall blocks. These blocks play a crucial role in infants and neonates, particularly in the paradigm shift toward minimally invasive and robotic surgeries. Table 9 illustrates the various fascial plane blocks and their indications, descriptions, and complications.
The penile block is frequently used for surgeries such as circumcision, meatoplasty, and hypospadias repair. It involves blocking the dorsal penile nerve and serves as a viable alternative to caudal epidural for the above-mentioned surgeries. In newborns, the most common indication is circumcision, a procedure exclusively feasible under this block. A 24 G short needle is used for this block. Complications include arterial puncture and the risk of ischemia. The use of adrenaline-containing solutions should be strictly avoided.
This block is mostly performed for postoperative pain management after palate surgeries in infants [38]. Recently, it has been advocated for tonsillectomies in older children.
The linear high-frequency probe should be positioned in the infra-zygomatic area, over the maxilla, with a 45° inclination in both frontal and horizontal planes [39]. The inclination should be adjusted based on the observed image to ensure clear visibility of the maxilla, sphenoid wing, and maxillary artery. The target destination for LA is the pterygopalatine fossa; the needle entry point is the junction of the lateral orbital rim and the zygomatic arch. The needle is advanced out of plane, and clear drug spread in the pterygopalatine fossa can be seen by a downward shift of the maxillary artery. A US-guided approach is superior to the landmark guidance [39].
The infraorbital block is commonly utilized in infants due to its efficacy in providing RA for cleft lip repair and orbital floor fracture repair [40]. This block involves injecting LA near the infraorbital foramen, which is situated on the maxillary bone below the orbit. The infraorbital nerve, a branch of the maxillary nerve, passes through this foramen, supplying sensation to the lower eyelid, upper lip, and part of the nasal ala.
The safety of RA in neonates and infants is well established, supported by numerous studies affirming its safety record. Nevertheless, it cannot be emphasized enough that practitioners must remain vigilant and exceptionally aware of potential complications in this vulnerable population. The most common complications with examples are depicted in Table 10.
Understanding and mastering local anesthetic systemic toxicity (LAST) in neonates and infants is paramount for the safe practice of RA. It is a critical aspect that cannot be overlooked in the pursuit of skillful administration in this vulnerable population. The management of the LAST is depicted in Fig. 7.
Successful management comprises an adequate perioperative pain regimen using multimodal analgesia along with adequate monitoring. Various age-appropriate pain assessment tools can be used to grade the severity of pain [41].
Multimodal analgesia, along with a rescue plan, is the best way to tackle pain. In practice, these strategies are often combined for optimal effect. For example, for a newborn undergoing a surgical procedure, a plan might include:
Preoperative: administration of paracetamol, 15–20 mg/kg as bolus dose, followed by 15 mg/kg per dose, thrice a day.
Intraoperative: use of local or RA to reduce the need for systemic opioids.
Postoperative: continued intravenous paracetamol or by suppository, with opioids available for breakthrough pain. Non-pharmacological strategies like swaddling, skin-to-skin contact, and sucrose can be used as adjuncts to soothe and comfort the infant when possible.
Nursing care and monitoring by a trained staff are the keys to improved perioperative care. For neuraxial blocks, hemodynamic monitoring, urinary retention, and block level should be evaluated. If peri-catheter leakage is detected, which is very common, the catheter need not be removed. Instead, pressure dressing and continuous infusion should be maintained after ruling out dislodgment of the catheter. Consider removal of the catheter only if the infusion fails to deliver clinically significant analgesia.
Soiling of the caudal epidural catheter with stool and urine is problematic. This issue can be mitigated by using water-resistant dressings or employing tunnelling techniques.
Preoperative communication between the surgeon, anesthesiologist, and parents about the surgery, anesthesia plan, and various risk factors should be routine, and doubts should be thoroughly discussed. Communication errors are expected during the handover time [42]. During the postoperative period, the surgical team, nursing staff, pediatric anesthesia specialists, and pain management teams should be in communication to ensure adequate pain management.
Recognizing the critical need for specialized expertise in pediatric RA, dedicated fellowships have become imperative. It is heartening that organizations like the Academy of Regional Anaesthesia India and the World Federation of Societies of Anaesthesiologists are addressing this need, offering specialized fellowships in our institution. These initiatives ensure a focused and advanced training ground, essential for the highest standards of care in pediatric RA. Simulation-based training can be the answer to many pitfalls like reduced exposure, caseload, and exposure hours, and is more effective than no intervention and non-inferior to non-simulation instruction [43]. Tele-simulation is also being tested for training and learning [44].
Overall, ensuring the safety, predictability, and effectiveness of pediatric RA treatment requires a systematic approach that includes thorough instruction in theory and hands-on practice under the guidance of an expert. It is crucial to reiterate that RA holds a definitive role in neonates and infants. A comprehensive understanding of the basics of anatomy, physiology, and their impact on block procedures, coupled with knowledge of local anesthetic pharmacology, is essential. Achieving this level of expertise demands dedicated efforts and meticulous honing of skills.
Notes
REFERENCES
2. Davidson AJ, Morton NS, Arnup SJ, De Graaff JC, Disma N, Withington DE, General Anesthesia compared to Spinal anesthesia (GAS) Consortium, et al. Apnea after Awake Regional and General Anesthesia in Infants: The General Anesthesia Compared to Spinal Anesthesia Study--Comparing Apnea and Neurodevelopmental Outcomes, a Randomized Controlled Trial. Anesthesiology. 2015; 123:38–54.
3. Subramaniam R. Anaesthetic concerns in preterm and term neonates. Indian J Anaesth. 2019; 63:771–9.

4. Puia-Dumitrescu M, Comstock BA, Li S, Heagerty PJ, Perez KM, Law JB, PENUT Consortium, et al. Assessment of 2-year neurodevelopmental outcomes in extremely preterm infants receiving opioids and benzodiazepines. JAMA Netw Open. 2021; 4:e2115998.

5. Barrington MJ, Kluger R. Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve blockade. Reg Anesth Pain Med. 2013; 38:289–9.

6. Ponde V, Singh N, Nair A, Ongaigui CJ, Nagdev T. Comparison of Landmark-guided, Nerve Stimulation-guided, and Ultrasound-guided Techniques for Pediatric Caudal Epidural Anesthesia: A Prospective Randomized Controlled Trial. Clin J Pain. 2021; 38:114–8.
7. Valairucha S, Seefelder C, Houck CS. Thoracic epidural catheters placed by the caudal route in infants: the importance of radiographic confirmation. Paediatr Anaesth. 2002; 12:424–8.

8. Ponde VC, Bedekar VV, Desai AP, Puranik KA. Does ultrasound guidance add accuracy to continuous caudal-epidural catheter placements in neonates and infants? Paediatr Anaesth. 2017; 27:1010–4.

9. Ponde V, Shah D, Nagdev T, Balasubramanian H, Boretsky K. Ultrasound determination of the dural sac to sacrococcygeal membrane distance in premature neonates. Reg Anesth Pain Med. 2022; 47:327–9.

10. Ponde VC, Bosenberg AT, Lokhandwala YY, Nagdev T, Puri K. Diagnostic and therapeutic application of thoracic epidural in a child with intractable cardiac arrhythmias: a case report. Paediatr Anaesth. 2022; 32:1073–5.

11. Ahmad N, Greenaway S. Anaesthesia for inguinal hernia repair in the newborn or ex-premature infant. BJA Educ. 2018; 18:211–7.

12. Verma D, Naithani U, Gokula C. Spinal anesthesia in infants and children: a one year prospective audit. Anesth Essays Res. 2014; 8:324–9.

13. Kokki H, Ylönen P, Laisalmi M, Heikkinen M, Reinikainen M. Isobaric ropivacaine 5 mg/ml for spinal anaesthesia in children. Anesth Analg. 2005; 100:66–70.
14. Vermeylen K, Engelen S, Sermeus L, Soetens F, Van de Velde M. Supraclavicular brachial plexus blocks: review and current practice. Acta Anaesthesiol Belg. 2012; 63:15–21.
16. Tsui BCH. Infraclavicular Brachial Plexus Block. Pediatric Atlas of Ultrasound- and Nerve Stimulation-Guided Regional Anesthesia. In : Tsui B, Suresh S, editors. New York: Springer;2016. p. 267–81.
17. Chin KJ, Alakkad H, Adhikary SD, Singh M. Infraclavicular brachial plexus block for regional anaesthesia of the lower arm. Cochrane Database Syst Rev. 2013; 28:CD005487.

18. Macfarlane AJ, Anderson KJ. Infraclavicular brachial plexus blocks. Continuing Education in Anaesthesia, Critical Care & Pain. 2009; 9:139–43.

19. Vedavyas R, Saravanan R, Mirunalini G, Gayathri B. A randomized controlled trial to compare the efficacy of single versus triple injection technique for ultrasound-guided infraclavicular block in upper limb surgeries. Local Reg Anesth. 2023; 16:51–8.

20. Ponde VC. Continuous infraclavicular brachial plexus block: a modified technique to better secure catheter position in infants and children. Anesth Analg. 2008; 106:94–6.

21. Ponde V, Athani B, Thruppal S. Infraclavicular coracoid approach brachial plexus block for radial club hand repair. Paediatr Anaesth. 2007; 17:863–6.

22. Ponde V, Singh N, Chavan D, Nagdev T. Interscalene brachial plexus block in a neonate: Here's how. Indian J Anaesth. 2021; 65:700–1.

23. Mahrous RSS, Ahmed AAA, Ahmed AMM. Comparison between ultrasound-guided caudal analgesia versus peripheral nerve blocks for lower limb surgeries in pediatrics: a randomized controlled prospective study. Local Reg Anesth. 2022; 15:77–86.

24. Munirama S, McLeod G. Ultrasound-guided femoral and sciatic nerve blocks. Continuing Education in Anaesthesia Critical Care & Pain. 2013; 13:136–40.

27. Ponde V, Desai AP, Shah D. Comparison of success rate of ultrasound-guided sciatic and femoral nerve block and neurostimulation in children with arthrogryposis multiplex congenita: a randomized clinical trial. Paediatr Anaesth. 2013; 23:74–8.

28. Ponde VC, Desai AP, Dhir S. Ultrasound-guided sciatic nerve block in infants and toddlers produces successful anesthesia regardless of the motor response. Paediatr Anaesth. 2010; 20:633–7.

29. Ponde VC, Desai AP, Shah DM, Johari AN. Feasibility and efficacy of placement of continuous sciatic perineural catheters solely under ultrasound guidance in children: a descriptive study. Pediatr Anesth. 2011; 21:406–10.

30. Alsaeed AH, Thallaj A, Khalil N, Almutaq N, Aljazaeri A. Ultrasound-guided rectus sheath block in children with umbilical hernia: case series. Saudi J Anaesth. 2013; 7:432–5.

31. Willschke H, Marhofer P, Bösenberg A, Johnston S, Wanzel O, Cox SG, et al. Ultrasonography for ilioinguinal/iliohypogastric nerve blocks in children. Br J Anaesth. 2005; 95:226–30.
32. Al-Sadek WM, Rizk SN, Selim MA. Ultrasound guided transversus abdominis plane block in pediatric patients undergoing laparoscopic surgery. Egyptian Journal of Anaesthesia. 2014; 30:273–8.

33. Hebbard P. Subcostal transversus abdominis plane block under ultrasound guidance. Anesth Analg. 2008; 106:674–5.

34. Hussein MM. Ultrasound-guided quadratus lumborum block in pediatrics: trans-muscular versus intra-muscular approach. J Anesth. 2018; 32:850–5.

35. Lucente M, Ragonesi G, Sanguigni M, Sbaraglia F, Vergari A, Lamacchia R, et al. Erector spinae plane block in children: a narrative review. Korean J Anesthesiol. 2022; 75:473–86.

36. Kandiah N, Walker K, Boretsky K. Ultrasound-guided paravertebral block facilitated tracheal extubation in a 5-week-old infant with rib fractures and respiratory failure. A A Case Rep. 2014; 2:131–2.

37. Vecchione T, Zurakowski D, Boretsky K. Thoracic paravertebral nerve blocks in pediatric patients: safety and clinical experience. Anesth Analg. 2016; 123:1588–90.
38. Smith L, Balakrishnan K, Pan S, Tsui BCH. Suprazygomatic maxillary (SZM) nerve blocks for perioperative pain control in pediatric tonsillectomy and adenoidectomy. J Clin Anesth. 2021; 71:110240.

39. Cao Z, Zhang K, Hu L, Pan J. Application of ultrasound guidance in the oral and maxillofacial nerve block. Peer J. 2021; 9:e12543.

40. Zdilla MJ, Russell ML, Koons AW. Infraorbital foramen location in the pediatric population: a guide for infraorbital nerve block. Paediatr Anaesth. 2018; 28:697–702.

41. Witt N, Coynor S, Edwards C, Bradshaw H. A guide to pain assessment and management in the neonate. Curr Emerg Hosp Med Rep. 2016; 4:1–10.

42. Bates DW, Singh H. Two decades since to err is human: an assessment of progress and emerging priorities in patient safety. Health Aff (Millwood). 2018; 37:1736–43.
43. Lorello GR, Cook DA, Johnson RL, Brydges R. Simulation-based training in anaesthesiology: a systematic review and meta-analysis. Br J Anaesth. 2014; 112:231–45.

Fig. 1.
(A) Position of the baby during caudal block. (B) Important landmarks to be noted during performing caudal block. PSIS: posterior superior iliac spine.
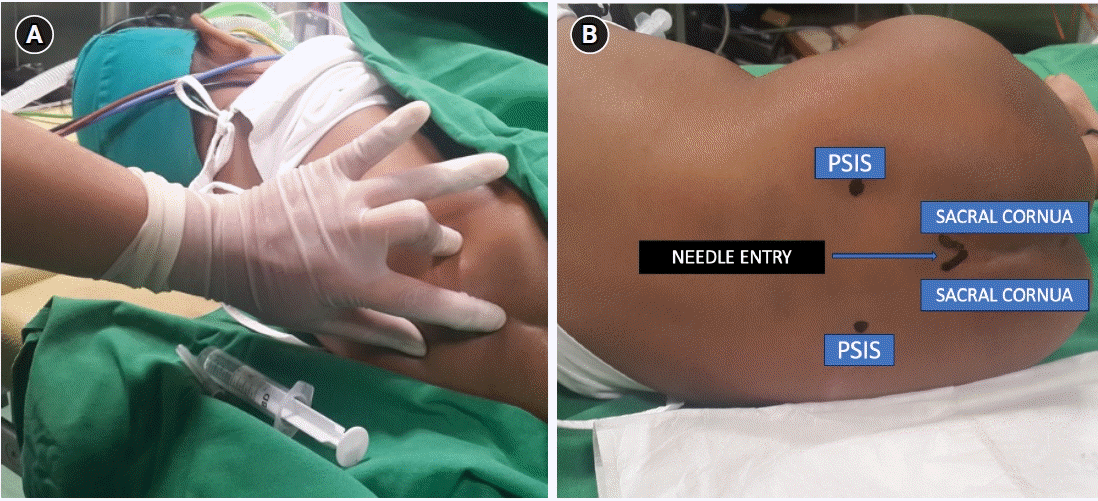
Fig. 2.
(A) Probe position in transverse orientation. (B) US image showing ‘Frogs eyes’ represented by the cornua of the sacrum. PSIS: posterior superior iliac spine, SC: sacral cornua.
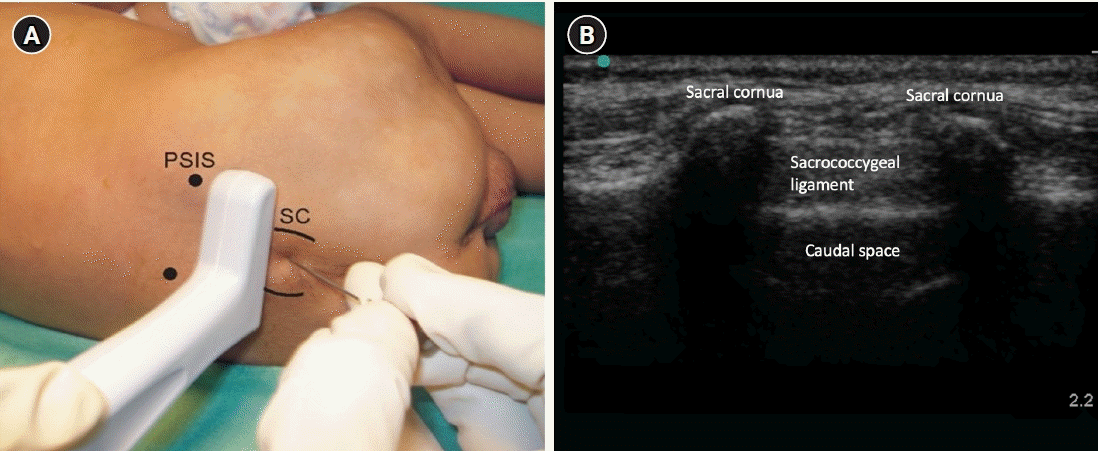
Fig. 3.
(A) Longitudinal scan showing the Sacrococcygeal ligament and caudal space. (B) shows needle entry piercing the ligament and needle tip in the caudal space. (C) shows the LA deposition in the caudal space and the anterior displacement of the posterior dura. LA: local anesthetic.
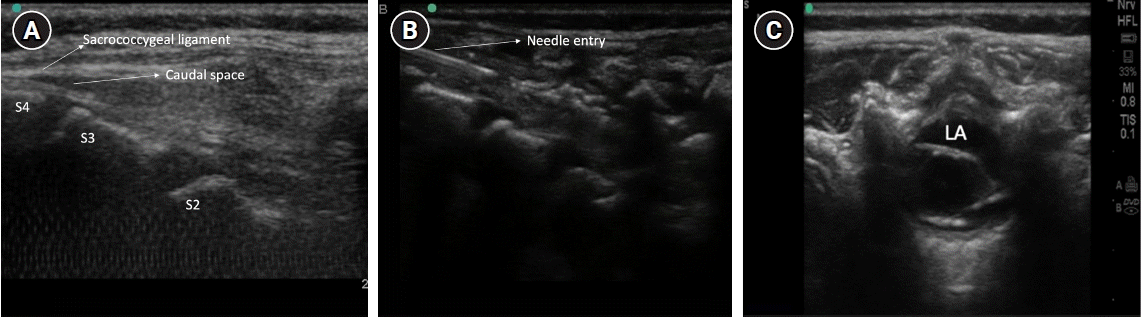
Fig. 4.
(A) The transverse scan of the central neuraxis at the level of thoracic level. (B) The various central neuraxial structure labelled at thoracic level. PVS: paravertebral space.
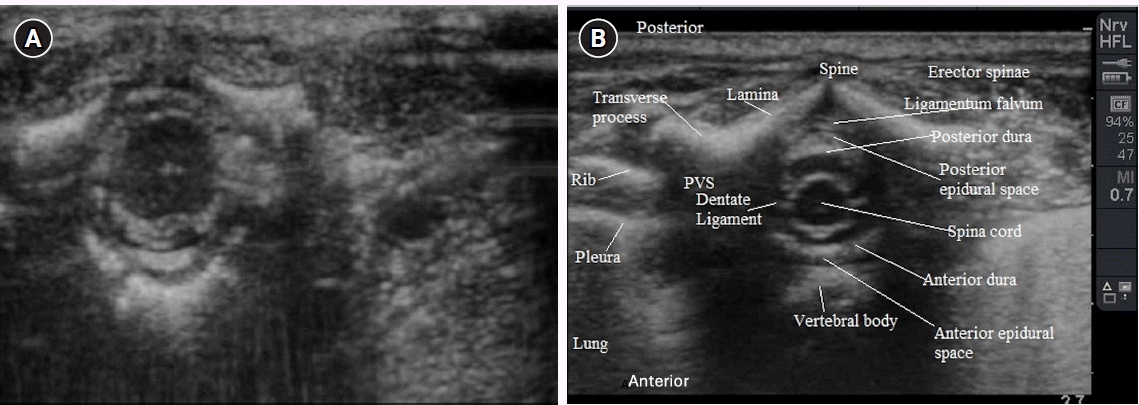
Fig. 5.
(A) Probe position during lateral infraclavicular block. (B) Ultrasonic image of lateral infraclavicular block showing the various cords of branchial plexus around the axillary artery. AA: axillary artery, AV: axillary vein, LC: lateral cord, MC: medial cord, PC: posterior cord.
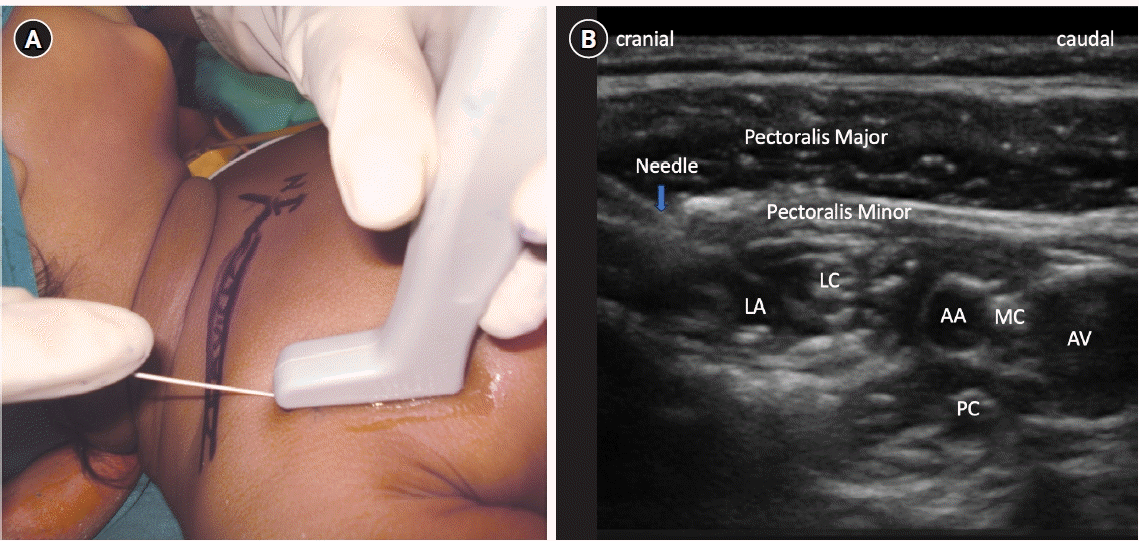
Fig. 6.
(A) Sonoanatomy of femoral nerve block. (B) Sonoanatomy of popliteal sciatic nerve block. FN: femoral nerve, FA: femoral artery, TN: tibial nerve, CPN: common peroneal nerve.
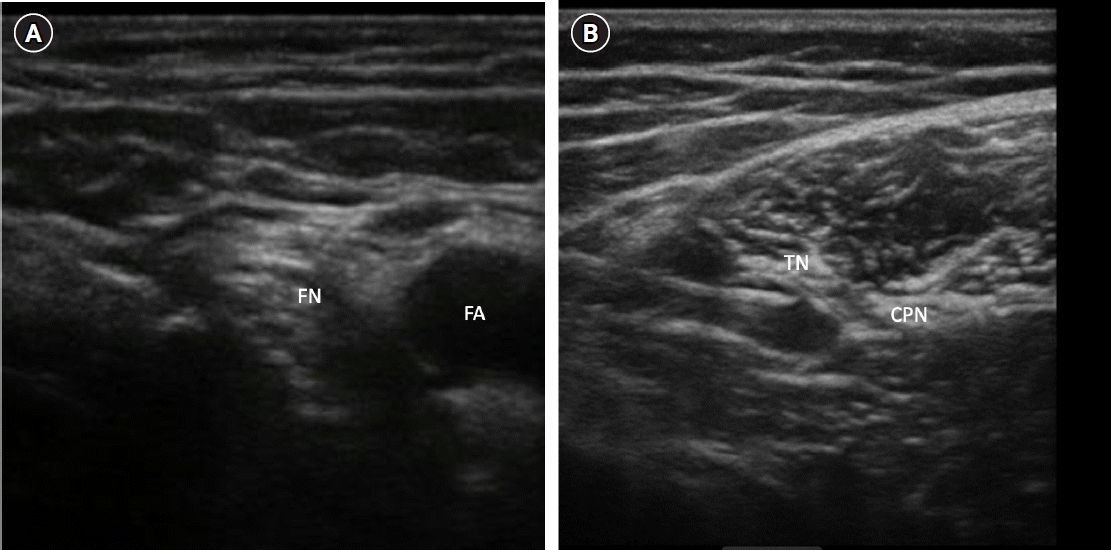
Fig. 7.
The management of the LAST. LAST: local anesthesia systemic toxicity, LA: local anesthetic, ACLS: advanced cardiac life support, CVS: cardiovascular system. *The dosage of epinephrine is restricted to 1 mcg/kg. Vasopressin should not be given. †Class 1 antiarrhythmics, beta blockers, calcium channel blockers are to be avoided. ‡The cumulative dose of intralipid infusion should not exceed 12 ml/kg.
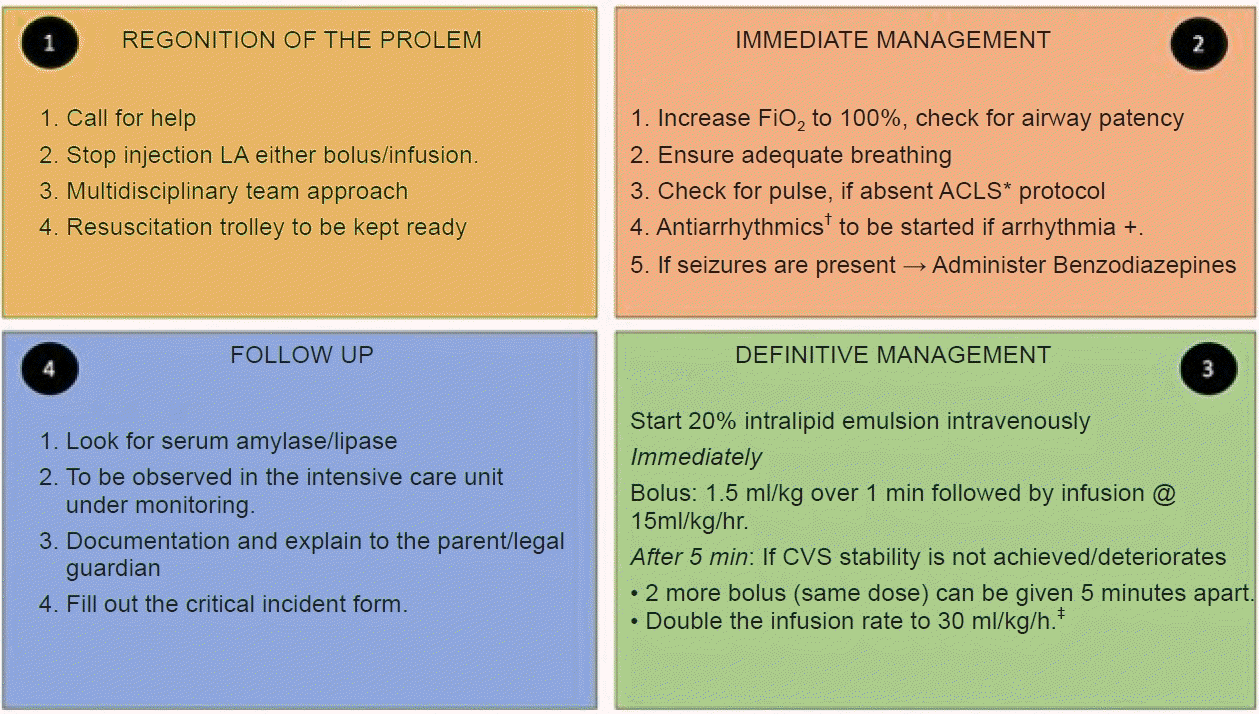
Table 1.
The Key Anatomical Differences in the Neonatal and Infant Central System and Their Clinical Implications
Table 2.
The Key Anatomical Differences in the Neonatal and Infant Nervous System, Vasculature and Their Clinical Implications
Table 3.
Key Physiological Differences and Its Anaesthetic Implication
| Physiological difference | Implications |
|---|---|
| Immature sympathetic nervous system and has parasympathetic dominance | Less hypotension secondary to SAB. |
| Vascular beds in the lower limbs are smaller accounting for lesser volume of venous pooling | |
| Immature hepatic function until 9 months of age coupled with high cardiac output. | High risk of LAST. |
| Reduced concentration of alpha-1 acid glycoprotein which binds to the LA | Increased level of unbound drugs in plasma |
| A higher proportion of cardiac sodium gated channels in an open state. | LA cardiotoxicity |
| They perceive more pain because of the lack/ill developed descending inhibitory pathway which modulates pain perception. | Good pain control is highly needed to prevent adverse neuro-behavioural outcome of chronic pain [45] |
| They poorly localise pain because of the greater receptive field of neuron. |
Table 4.
The Various Common LA Options and Dosages [15]
Table 5.
The Adjuvants Used and Dosages [15]
Table 6.
Various Possible Positions of the Needle Followed by Inference, Implications, and Troubleshooting
Table 7.
Common Issues with Continuous Caudal Epidural Catheters in Neonates and Infants
Table 8.
Commonly Used Drug and Dosages in Lumbar and Thoracic Epidurals in Neonates and Infants
Table 9.
Illustrates the Various Fascial Plane Blocks, Along with Their Indication, Descriptions, and Complications
| Block | Description | Indications | Complications and considerations |
|---|---|---|---|
| Rectus sheath block [30] | Bilateral blocks covering anaesthesia from the central aspect of the anterior abdominal wall to the iliac crest. | Umbilical surgeries | Peritoneal puncture; risk of intra-abdominal injury mandatory ultrasound guidance |
| Suitable for umbilical surgeries. | |||
| Effective. | |||
| Ilioinguinal and Iliohypogastric nerve block [31] | Provides analgesia for inguinal surgeries. | Inguinal surgeries | Difficulty in tissue plane identification. No anaesthesia for hernial sac |
| TAP block [32] | Offers analgesia to the ipsilateral anterolateral abdominal wall. | MIS | Risk of intra-abdominal injury crucial in multimodal analgesia for MIS. |
| Subcostal TAP block [33] | Targets the ipsilateral upper quadrant. | Upper quadrant surgeries | Risk of intra-abdominal injury |
| Quadratus Lumborum block [34] | Indicated in various surgeries, providing somatic and visceral analgesia to the ipsilateral side. | Lower abdominal and hip surgeries | Kidney injury; injury to lumbar artery. |
| Lingering motor block | |||
| Erector spinae plane block [35] | Promises somatic analgesia for unilateral abdominal and thoracic procedures. Further clinical evaluation needed for visceral analgesia | Unilateral abdominal and thoracic procedures | Further clinical evaluation needed |
| Paravertebral block [36,37] | Offers somatosensory analgesia for unilateral thorax and trunk surgeries. USG enhances safety. | Thorax and trunk surgeries | Hypotension; vascular puncture; pleural puncture; pneumothorax. |
| Not for use in lung or pleura infective conditions | |||
| Interpectoral plane block and Pecto-serratus plane block, Serratus anterior plane blocks | Alternatives for thoracic epidural, paravertebral, intercostal, and intrapleural blocks. | Intercostal drain insertion or surgeries over the hemithorax in children | Continuous catheter placement is difficult to place and maintain for major thoracic surgeries. |
| Provides analgesia for intercostal drain insertion or surgeries over the hemithorax in children. |
Table 10.
The Most Common Complications with Examples




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download