Abstract
The optimal oxygen target during general anesthesia remains difficult to define in pediatric and adult patients. Although access to pediatric patients has become difficult owing to a decrease in birth rate, pediatric anesthesia remains an important part of anesthesiology, and oxygenation related to general anesthesia is an essential part of any anesthesiologist. The use of oxygen has increased survival rates in adults and children; however, the side effects related to oxygen use have also increased. This review addresses the considerations of oxygenation in pediatric patients undergoing general anesthesia.
Oxygen is present in humans and is essential for the existence of not only humans but also all life on Earth. Maintaining a certain concentration of this gas in the body of life on Earth is essential for living. Physiological changes outside the normal range can cause serious pathological conditions [1]. Anesthesiologists are accustomed to dealing with oxygen, but it is sometimes difficult to approach its use in children because of limited training and lack of experience, owing to the decline in the pediatric population due to low birth rates. In addition, it is important to understand the differences between children and adults because children show different anatomical and physiological characteristics [2]. Although the number of pediatric patients receiving general anesthesia has decreased, according to the results of the Korea Insurance Review and Assessment Service, 81,243 pediatric patients, which accounted for approximately 10% of the total number of patients as of 2022, received general anesthesia requiring endotracheal intubation. Therefore, pediatric anesthesia remains an important factor for anesthesiologists need to know.
As the use of oxygen has historically been around for a long time, various equipment and manipulations have been developed along with oxygen use, which has contributed to increasing the survival of children with difficult airways [3-5]. In addition, several studies have been conducted on complications caused by the use and concentration of oxygen [6-8]. However, we remain unsure what the optimal target of oxygen is during anesthesia in pediatric patients. Therefore, it is hoped that this short review will provide an opportunity to consider perioperative optimal oxygen levels in children.
The use of oxygen for anesthesia in modern medicine can be traced back to the 1800s [9]. Unlike the time when it was only focused on simply anesthetizing the patient, modern anesthesia includes not only a series of processes to safely induce, maintain, and restore anesthesia to patients but also everything to care for patients during the perioperative period [10,11]. Moreover, the introduction of a pulse oximeter in the 1970s provided an opportunity to recognize the danger of hypoxia along with failure of tracheal intubation and brought one step closer to safe anesthesia [12,13]. Although the use of oxygen has prevented hypoxia and increased survival in patients with difficult tracheal intubation, the use of oxygen under the manipulation of anesthesia caused other complications, which led anesthesiologists to consider the concept of an appropriate oxygen concentration [14].
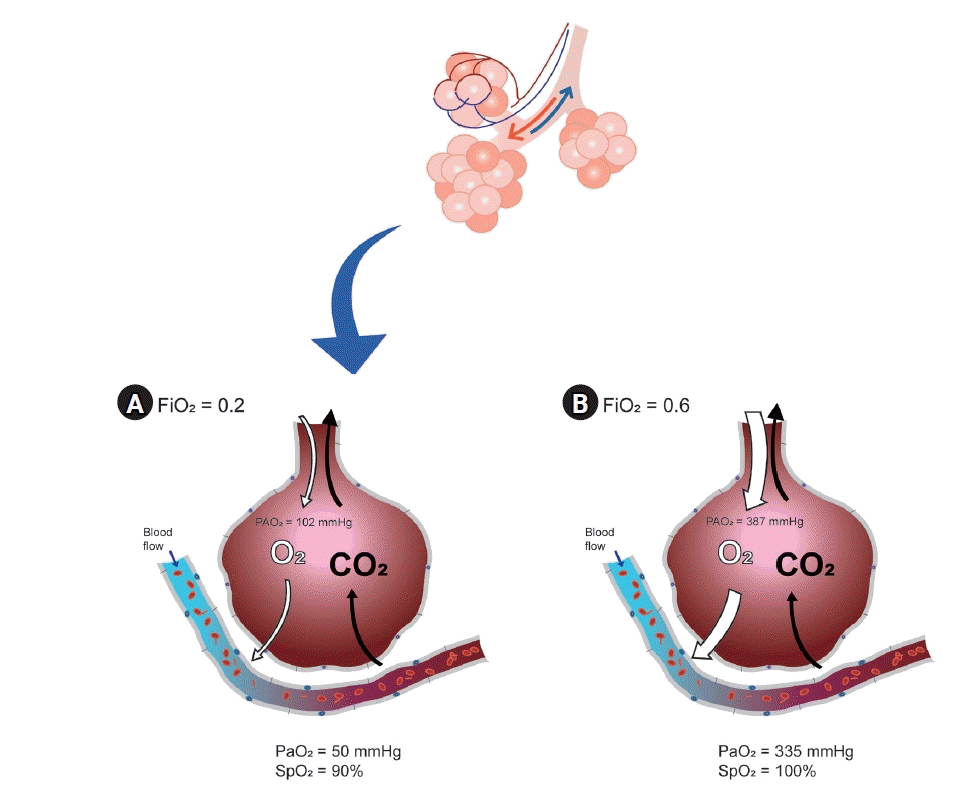
Optimal oxygen is usually defined as the optimal arterial oxygen tension (PaO2) at the lowest inspired oxygen concentration (FiO2) [15]. Ultimately, it would be the purpose of using sufficient oxygen to maximize the effects and reduce the side effects. In situations where arterial blood gas analysis (ABGA) can be performed, the target value can be obtained by adjusting the ventilation strategy as needed, after measuring PaO2 and comparing the result to FiO2 to evaluate the alveolar to arterial oxygen (A-a) gradient. If ABGA are not available, pulse oximetry is the next most popular method for oxygenation assessment. Everyone knows that the pulse oximetry is important; however, it is not the perfect monitoring device for problems with oxygen exchange in the lungs when FiO2 is not restricted. A pulse oximeter can measure arterial oxyhemoglobin saturation (SpO2), but not arterial partial pressure (PaO2), if FiO2 is more than 21%. Even when there is a serious problem in the lungs, oxygen saturation expressed by a pulse oximeter can be 100% (Fig. 1) [15]. Pulse oximetry can confirm hypoxia and related events but cannot reduce mortality [13]. In addition, a pulse oximeter cannot confirm the grade of hyperoxia because SpO2 is only sigmoidally correlated with a state within less than 100 mmHg of PaO2 [16]. Recently, a new monitoring device called the oxygen reserve index was introduced; however, if an accurate evaluation of the oxygen exchange problem is required, ABGA is the most important [17-19].
As such, it is difficult to understand the optimal oxygen level itself, but there are many aspects to consider regarding optimal oxygen for pediatric patients. Unlike adults, children continue to grow from the moment they are fertilized, and everything we do can affect them. Additionally, this range varies depending on their pathology.
Compared with adults, children have higher oxygen consumption owing to increased metabolism and immature breathing [20-22]. Moreover, the more severe these symptoms are, the higher the possibility of postoperative apnea [21]. In addition, the diameter of the airway is small and it collapses easily, resulting in a weak pharyngeal muscle tone. Breathing mainly occurs through the nose, with increased resistance in the upper and lower airways [23]. These changes can increase the work of breathing. In addition, not only is the lung volume small because of the small number of alveoli and underdeveloped collateral ventilation, but the efficiency of the respiratory structures (diaphragm, intercostal muscle, and ribs) is also low [22,24-26]. In addition to these basic differences, changes in growth cannot be ignored.
Even at the moment of birth, it continues to grow. In an environment different from that of the fetus, basic ABGA levels are completely different from those of adults. To inflate the fluid-filled lungs of the fetus, neonates overcome large surface forces in the first few breaths, typically requiring negative pressures of 30 cmH20 [26]. As a result, a period of transient hypoxia, hypercapnia, and acidemia, and changes in normal ABGA values occur [26,27].
Various factors affect oxygen delivery to tissues; not only the function of the lungs but also the role of mediators that transport and deliver oxygen is important [28,29]. As is commonly known, fetal hemoglobin has a higher affinity for oxygen than adult hemoglobin, and less oxygen is delivered to surrounding tissues [30]. The p50 (the level of PaO2 that becomes SaO2 50%, which indicates the affinity of hemoglobin for oxygen) used to express this affinity is 18-19 mmHg in newborns, whereas it is higher at 27 mmHg in adults. [31,32]. After birth, the total hemoglobin levels decrease rapidly, resulting in physiological anemia at 2 or 3 months [33]. During this period, p50 also increases, reaching its highest value at 10 months and maintaining it for 10 years [34]. This means that oxygen transport in infants and children is as effective as in adults, although infants and children have lower hemoglobin levels compared to high hemoglobin in adults. However, there is still much controversy regarding what happens to normal PaO2 and SaO2 that should be maintained in pediatric patients. In particular, it is more difficult to determine whether a patient is under anesthesia and has various pathologies.
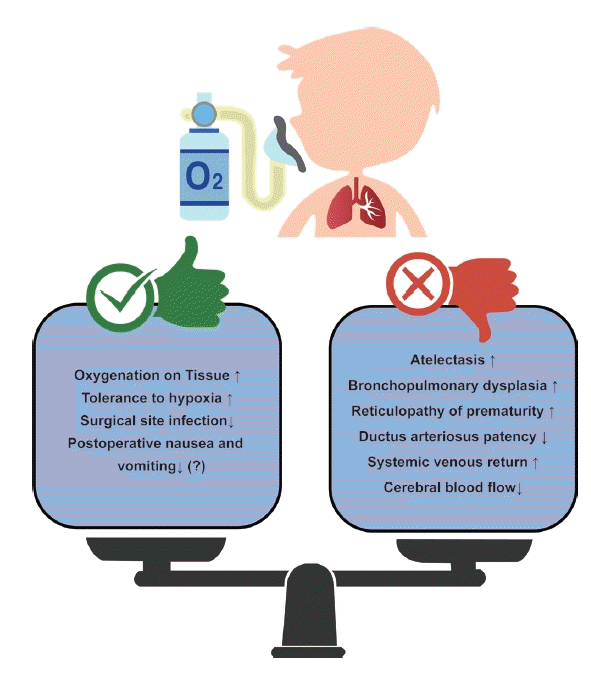
In pediatric departments, which usually deal with pathological conditions in children, oxygen is used to treat hypoxia. However, anesthesia differs from the awakened state. Most patients who undergo surgery under general anesthesia have normal respiratory conditions. Hypoxia in the anesthesia department is a complication of anesthesia. Therefore, the purpose of the oxygen supply in the anesthesia department is to ensure proper oxygenation before, during, and after surgery. Regardless of whether the method is mechanical ventilation or spontaneous ventilation, the purpose is to properly oxygenate tissues before, during, and after surgery and to minimize complications in the perioperative period. Therefore, prophylactically administered oxygen should be administered with a different strategy than that of therapeutically administered oxygen. In the case of hypoxia or oxygen diffusion disorders, supplying a high concentration of oxygen is a treatment; however, it is not a method to prevent hypoxemia due to the ventilation/perfusion (V/Q) mismatch that occurs mostly during surgery [35,36].
In particular, unlike adults, the infant respiratory system maintains a balance between the chest expansion force and lung contraction force through a highly compliant chest and increased lung elastic recoil. In contrast, the provision of intraoperative hyperoxia under these conditions disrupts this balance, leading to a decrease in functional residual capacity, a greater propensity for airway collapse, reduced lung volume, and subsequent hypoxemia [37,38]. Therefore, the induction and maintenance of anesthesia with 100% oxygen can significantly reduce the lung volume participating in gas exchange and cause airway obstruction [39]. In addition, many V/Q mismatches occur in children owing to a decrease in lung volume. Using a high concentration of oxygen, the area where atelectasis has occurred can be masked and SaO2 can be maintained at a high level [37,38]. Several studies have confirmed that using low rather than high concentrations of oxygen can reduce atelectasis and maintain lung volume [40,41]. If attending anesthesiologists would like to check when intraoperative alveolar closure occurs, it is better to maintain the FiO2 at 30–35% during surgery [37].
Oxygen does not have only advantages. Opinions on which the optimal oxygen level was still divided (Fig. 2). As mentioned previously, when a high concentration of oxygen is used, absorption atelectasis can be caused by airway closure and collapse [42]. It can also cause interactions with the cardiovascular system, such as an increase in pulmonary vascular resistance, an increase in pulmonary circulation, and a decrease in cerebral blood flow because of systemic artery constriction and cerebral vessel constriction [43,44]. In addition, oxygen toxicity exists, which is caused by reactive oxygen metabolites that can particularly affect preterm or newborn babies [45]. Oxidative stress caused by the production of reactive oxygen metabolites alters macromolecules such as deoxyribonucleic acid (DNA) and proteins, causing epithelial and endothelial cell damage, increasing capillary permeability that promotes the passage of cytokines, and causing inflammation and edema, resulting in bronchopulmonary dysplasia (BPD) [37,46]. Oxygen can also cause retinopathy of prematurity (ROP), in which fibrovascular proliferation occurs without normal blood vessel development [47,48].
In particular, the lastly mentioned BPD or ROP is related to studies on oxygen targeting in newborns or premature babies, which have been conducted for a long time. According to various studies, if the SpO2 target is set low, even in premature infants, the incidence of retinopathy of prematurity is reduced, but the mortality rate increases (low target 85–89% and high target 91–95%) [49-52]. However, this study was conducted on critically ill children in the neonatal intensive care unit, and there are limitations to its application in healthy children undergoing surgery in a short time. In a recent study of healthy children divided into hypoxia and hyperoxia groups, transcutaneous oxygen and SpO2 measured by pulse oximetry were significantly lower in the hypoxia group; however, regional cerebral oxygen saturation (rSO2) showed no difference between the two groups. Additional research is needed to determine whether hyperoxia is as dangerous as hypoxia, but the rSO2 value decreased regardless of FiO2 in this study, and it seems to reflect the pharmacological effect of an anesthetic induction agent, which additional research is needed [53].
In addition to newborns and premature babies, the representative patients are children with congenital heart disease (CHD) among pediatric patients who require caution when administering oxygen. In the case of CHD, there are many factors to consider, but among them, oxygenation can give affect or be affected by shunts caused by defects in the heart. It is important to accurately determine the patient's condition because the degree of influence of oxygen and results from oxygen varies depending on the size of the shunt, direction of the shunt, and the presence or absence of a patent ductus arteriosus. In acyanotic CHD, supplementation with high concentrations of oxygen increases systemic vascular resistance and decreases the cardiac index, systemic oxygen delivery [54]. In particular, in children with single-ventricle physiology, excessive oxygen causes hemodynamic instability, and insufficient oxygen causes hypoxia, acidosis, and neurological damage. In addition, in ductus-dependent CHD patients such as those with hypoplastic left heart syndrome, if oxygen is used excessively despite the administration of prostaglandins, systemic circulation is disturbed by increased pulmonary circulation and the ductus arteriosus can become blocked and unstable. Therefore, it is important to maintain SpO2 at 75–85%, and it is desirable to carefully administer oxygen in consideration of various situations [37,55].
According to the World Health Organization (WHO) guidelines for perioperative oxygen in adults, the use of a high concentration of oxygen has significant advantages over the use of a low concentration of oxygen in terms of prevention of surgical site infection; however, this guideline has limitations in that anesthesiologists were not included to make this guideline and there is no opinion on children [56,57]. Even though a meta-analysis is expected to be performed using a recently published protocol, it is questionable how many children will be included in this study [58]. In addition, postoperative nausea and vomiting can decrease in adults with higher oxygen levels than in those with lower oxygen levels, but not in pediatrics [59-61]. Although there are different opinions, it is certain that it would be best to avoid hypoxia and hyperoxia. The optimal target of oxygenation during general anesthesia in pediatric patients cannot be defined as a single unified range, and additional evidence is required to define it. Therefore, anesthesiologists should consider the various conditions of pediatric patients before, during, and after surgery for optimal oxygenation in pediatric patients undergoing general anesthesia.
REFERENCES
1. Ortiz-Prado E, Dunn JF, Vasconez J, Castillo D, Viscor G. Partial pressure of oxygen in the human body: a general review. Am J Blood Res. 2019; 9:1–14.
2. Sen I, Dave N, Bhardwaj N, Juwarkar C, Beegum S. Specialised training in paediatric anaesthesia: Need of the hour. Indian J Anaesth. 2021; 65:17–22.
3. Law JA, Broemling N, Cooper RM, Drolet P, Duggan LV, Griesdale DE, et al. The difficult airway with recommendations for management--part 2--the anticipated difficult airway. Can J Anaesth. 2013; 60:1119–38.
4. Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology. 2022; 136:31–81.

5. Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, Difficult Airway Society intubation guidelines working group, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015; 115:827–48.

6. Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981; 256:10986–92.

9. Smith WD. A history of nitrous oxide and oxygen anaesthesia. Part V: The crucial experiment, its eclipse, and its revival. Br J Anaesth. 1966; 38:143–56.

10. Kain ZN, Fitch JCK, Kirsch JR, Mets B, Pearl RG. Future of anesthesiology is perioperative medicine: a call for action. Anesthesiology. 2015; 122:1192–5.
11. Dean HF, Carter F, Francis NK. Modern perioperative medicine - past, present, and future. Innov Surg Sci. 2019; 4:123–31.

12. Van Meter A, Williams U, Zavala A, Kee J, Rebello E, Tsai J, et al. Beat to beat: a measured look at the history of pulse oximetry. J Anesth Hist. 2017; 3:24–6.

13. Pedersen T, Nicholson A, Hovhannisyan K, Moller AM, Smith AF, Lewis SR. Pulse oximetry for perioperative monitoring. Cochrane Database Syst Rev. 2014; 2014:CD002013.

14. Shykoff BE, Lee RL. Risks from breathing elevated oxygen. Aerosp Med Hum Perform. 2019; 90:1041–9.

15. Feldman JM. Optimal ventilation of the anesthetized pediatric patient. Anesth Analg. 2015; 120:165–75.

17. Scheeren TWL, Belda FJ, Perel A. The oxygen reserve index (ORI): a new tool to monitor oxygen therapy. J Clin Monit Comput. 2018; 32:379–89.

18. Scheeren TWL, Belda FJ, Perel A. Correction to: The oxygen reserve index (ORI): a new tool to monitor oxygen therapy. J Clin Monit Comput. 2018; 32:579–80.

19. Ishida Y, Okada T, Kobayashi T, Uchino H. ORiTM: a new indicator of oxygenation. J Anesth. 2021; 35:734–40.
20. Lindahl SG. Oxygen consumption and carbon dioxide elimination in infants and children during anaesthesia and surgery. Br J Anaesth. 1989; 62:70–6.

22. Trachsel D, Erb TO, Hammer J, Von Ungern-Sternberg BS. Developmental respiratory physiology. Paediatr Anaesth. 2022; 32:108–17.

23. Adewale L. Anatomy and assessment of the pediatric airway. Paediatr Anaesth. 2009; 19 Suppl 1:1–8.

24. Widdicombe JG, Nadel JA. Airway volume, airway resistance, and work and force of breathing: theory. J Appl Physiol. 1963; 18:863–8.

26. Saikia D, Mahanta B. Cardiovascular and respiratory physiology in children. Indian J Anaesth. 2019; 63:690–7.

27. Dawes GS. The central control of fetal breathing and skeletal muscle movements. J Physiol. 1984; 346:1–18.

30. Pritisanac E, Urlesberger B, Schwaberger B, Pichler G. Fetal hemoglobin and tissue oxygenation measured with near-infrared spectroscopy-a systematic qualitative review. Front Pediatr. 2021; 9:710465.
31. Thomas C, Lumb AB. Physiology of haemoglobin. Continuing Education in Anaesthesia Critical Care & Pain. 2012; 12:251–6.

32. Vali P, Underwood M, Lakshminrusimha S. Hemoglobin oxygen saturation targets in the neonatal intensive care unit: Is there a light at the end of the tunnel? Can J Physiol Pharmacol. 2019; 97:174–82.

33. O'Brien RT, Pearson HA. Physiologic anemia of the newborn infant. J Pediatr. 1971; 79:132–8.
35. Trachsel D, Svendsen J, Erb TO, Von Ungern-Sternberg BS. Effects of anaesthesia on paediatric lung function. Br J Anaesth. 2016; 117:151–63.

36. Lumb AB. Why do patients need extra oxygen during a general anaesthetic? BJA Educ. 2019; 19:37–9.

37. Habre W, Peták F. Perioperative use of oxygen: variabilities across age. Br J Anaesth. 2014; 113 Suppl 2:ii26–36.

39. Von Ungern-Sternberg BS, Regli A, Schibler A, Hammer J, Frei FJ, Erb TO. The impact of positive end-expiratory pressure on functional residual capacity and ventilation homogeneity impairment in anesthetized children exposed to high levels of inspired oxygen. Anesth Analg. 2007; 104:1364–8.

40. Kim HI, Min JY, Lee JR, Kwan Woong C, Cho MR, Byon HJ. The effect of oxygen concentration on atelectasis formation during induction of general anesthesia in children: A prospective randomized controlled trial. Paediatr Anaesth. 2021; 31:1276–81.

41. Grandville B, Petak F, Albu G, Bayat S, Pichon I, Habre W. High inspired oxygen fraction impairs lung volume and ventilation heterogeneity in healthy children: a double-blind randomised controlled trial. Br J Anaesth. 2019; 122:682–91.
42. O'Brien J. Absorption atelectasis: incidence and clinical implications. AANA J. 2013; 81:205–8.
43. Green S, Stuart D. Oxygen therapy for pulmonary arterial hypertension: We need to rethink and investigate. Respirology. 2020; 25:470–1.

45. Jamieson D. Oxygen toxicity and reactive oxygen metabolites in mammals. Free Radic Biol Med. 1989; 7:87–108.

46. Perrone S, Tataranno ML, Buonocore G. Oxidative stress and bronchopulmonary dysplasia. J Clin Neonatol. 2012; 1:109–14.

47. Philip AG. Oxygen plus pressure plus time: the etiology of bronchopulmonary dysplasia. Pediatrics. 1975; 55:44–50.

48. Hartnett ME, Lane RH. Effects of oxygen on the development and severity of retinopathy of prematurity. J AAPOS. 2013; 17:229–34.
49. Group BIUKC, Group BIAC, Group BINZC, Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013; 368:2094–104.

50. Lakshminrusimha S, Manja V, Mathew B, Suresh GK. Oxygen targeting in preterm infants: a physiological interpretation. J Perinatol. 2015; 35:8–15.

51. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010; 362:1959–69.

52. Darlow BA, Marschner SL, Donoghoe M, Battin MR, Broadbent RS, Elder MJ, et al. Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. J Pediatr. 2014; 165:30–5.e2.

53. Karlsson V, Sporre B, Fredén F, Ågren J. Randomized controlled trial of low vs high oxygen during neonatal anesthesia: Oxygenation, feasibility, and oxidative stress. Paediatr Anaesth. 2022; 32:1062–9.

54. Beekman RH, Rocchini AP, Rosenthal A. Cardiovascular effects of breathing 95 percent oxygen in children with congenital heart disease. Am J Cardiol. 1983; 52:106–11.

55. Shivananda S, Kirsh J, Whyte HE, Muthalally K, McNamara PJ. Impact of oxygen saturation targets and oxygen therapy during the transport of neonates with clinically suspected congenital heart disease. Neonatology. 2010; 97:154–62.

56. Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, De Jonge S, De Vries F, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016; 16:e288–303.

57. Volk T, Peters J, Sessler DI. The WHO recommendation for 80% perioperative oxygen is poorly justified. Anaesthesist. 2017; 66:227–9.

58. Elfeky A, Chen YF, Grove A, Hooper A, Wilson A, Couper K, et al. Perioperative oxygen therapy: a protocol for an overview of systematic reviews and meta-analyses. Syst Rev. 2022; 11:140.

59. Behera BK, Misra S, Mohanty MK, Srinivasan A. Effect of intra-operative high inspired fraction of oxygen on postoperative nausea and vomiting in children undergoing surgery: A prospective randomised double-blind study. Eur J Anaesthesiol. 2021; 38:1124–9.
Fig. 1.
Different oxygenation in the same patient who have lung exchange problem depending on the fraction inspired oxygen concentration: (A) with lower inspired oxygen, (B) with higher inspired oxygen. CO2: carbon dioxide, FiO2: fraction of inspired oxygen, O2: oxygen, PAO2: alveolar oxygen partial pressure, PaO2: partial pressure of oxygen in arterial blood, SpO2: peripheral oxygen saturation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download