Abstract
Recently, a paradigm shift has occurred in the classification of diverticular disease and the understanding of its pathogenesis. Diverticular disease is now defined as a variety of clinically significant conditions such as diverticulitis, diverticular bleeding, symptomatic uncomplicated diverticular disease, and segmental colitis associated with diverticulosis. Low-grade inflammation, visceral hypersensitivity, abnormal intestinal motility, and genetic factors have emerged as the key contributors to the pathogenesis of diverticular disease. Routine antibiotic use is no longer recommended for all cases of diverticulitis, and simple recurrence is not an indication for surgical treatment. Early colonoscopy with proper preparation is recommended for the treatment of diverticular bleeding, although recent studies have not shown significant efficacy in preventing recurrence. The roles of dietary fiber, nonabsorbable antibiotics, 5-aminosalicylates, and probiotics in the prevention of diverticular disease are controversial and require further investigation.
Colonic diverticulosis is defined as a protrusion of the mucosa and submucosa through a weak portion of the muscle layer [1]. Although the underlying mechanism of diverticulosis has not been fully elucidated, decreased stool volume due to a low-fiber diet, delayed colon transit, constipation, and increased intraluminal pressure due to overcontraction have been implicated [2-4].
Although the prevalence of colonic diverticulosis is lower in Asia than in Western countries, it has increased with an aging society and westernized food culture [5]. Diverticular disease is sometimes considered synonymous with diverticulosis; however, the exact definition of diverticular disease is a condition that combines clinically significant symptoms, including diverticulitis and diverticular bleeding [6-8]. In this review, we discuss the classification and unresolved issues associated with diverticular diseases in terms of diagnosis, treatment, and prevention.
Diverticulosis can be classified as asymptomatic or symptomatic. Symptomatic diverticulosis is similar to diverticular disease. Asymptomatic diverticulosis is usually detected incidentally during radiologic or colonoscopic evaluation [1]. According to a previous report, diverticulosis is more common in the left colon in Western countries, and not all colonic walls protrude (pseudodiverticulum) [1]. In contrast, in Asia, diverticulosis usually presents as a true diverticulum, which is more common in the right colon and does not increase with aging [1]. However, these differences decrease over time in Asians who move to Western countries, indicating the significant impact of diet and lifestyle [9].
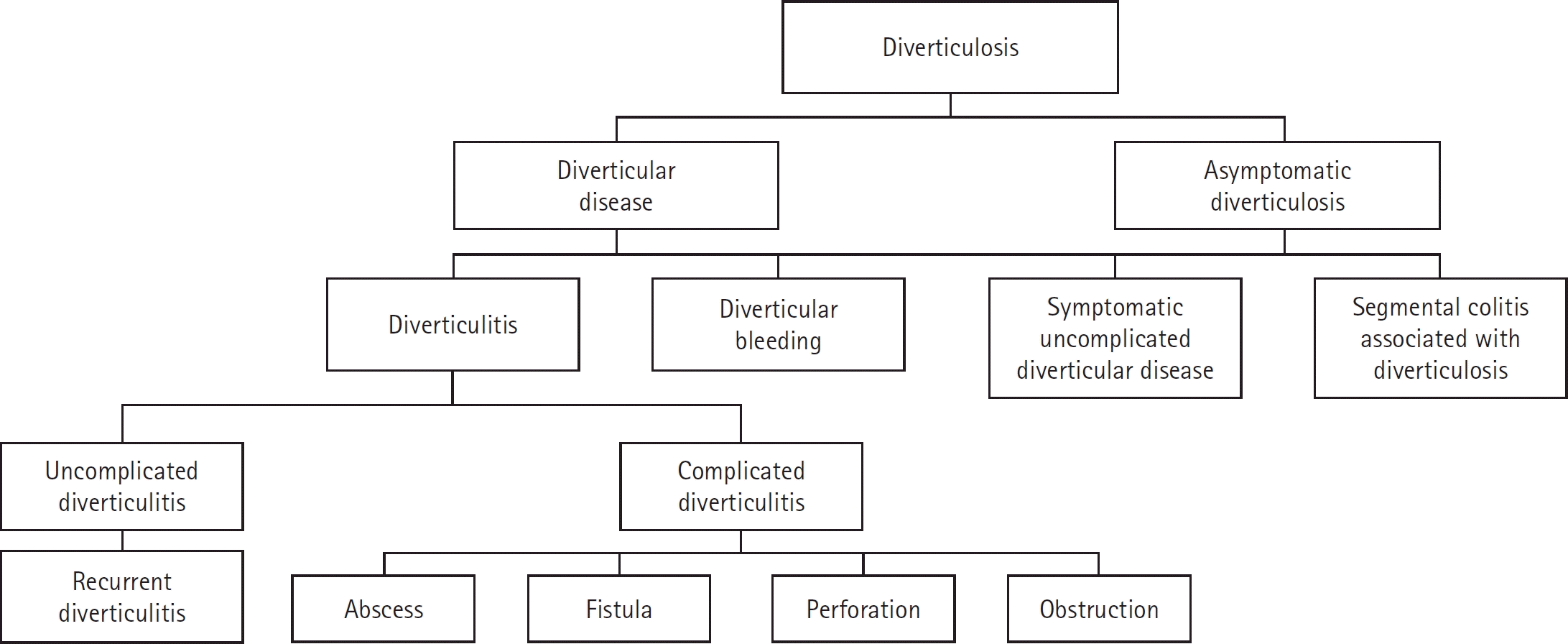
A precise definition of the clinical significance of asymptomatic diverticulosis is lacking, making indications for treatment controversial [10]. Symptomatic diverticulosis or diverticular disease includes diverticulitis, diverticular bleeding, symptomatic uncomplicated diverticular disease (SUDD), and segmental colitis associated with diverticulosis (SCAD) (Fig. 1) [9].
Diverticulitis can be defined as acute or chronic inflammation of the diverticulum and is the most common complication (4%–15%) of diverticulosis [11,12]. Diverticulitis develops when fecaliths obstruct the sac owing to mucosal irritation, low-grade inflammation, and congestion [10]. Diverticulitis can be classified based on the presence or absence of complications [13]. Uncomplicated diverticulitis is an inflammation of the diverticulum and its surrounding wall, whereas complicated diverticulitis presents with an abscess, perforation, fistula, stricture, or obstruction. Diverticulitis has a prevalence of 12% to 15% [14,15].
Diverticular bleeding is one of the main causes of acute lower gastrointestinal bleeding. According to a previous study, approximately 30% to 65% of lower gastrointestinal bleeding cases are related to diverticula [18]. A bleeding incidence of 10% to 15% has been reported in patients with diverticulosis [7,8,19]. Bleeding is thought to develop from the herniated exposed vasa recta that protrudes over the dome of the diverticulum [1]. Diverticular bleeding is more common in the right-sided colon because the wall is thinner and the neck of the diverticulum is wider [20-22].
Diverticular bleeding is usually painless and is self-limiting in most cases [23]. Bleeding is brisk in some patients with hypotension and tachycardia, requiring resuscitation and transfusion.
SUDD is suspected when patients complain of nonspecific abdominal pain with no evidence of inflammation in the diverticulum by endoscopy or radiology [1]. Pain is typically colicky but can be continuous and improves after defecation. Changes in bloating and bowel habits may also occur. Considering these symptoms, distinguishing SUDD from irritable bowel syndrome (IBS) can be challenging. A recent study reported a prevalence of up to 15% for SUDD in diverticulosis, with classifications including SUDD, IBS-like SUDD, and post-diverticulitis SUDD [25].
The suggested pathogenesis of SUDD includes visceral hypersensitivity, low-grade inflammation, changes in gut microbiota, and abnormal colonic motility following an episode of diverticulitis [26-30]. Due to their overlapping pathogeneses and clinical symptoms, the current treatments for SUDD and IBS are similar, including fiber intake and antibiotics such as rifaximin [31,32]. Mesalamine, probiotics, and antispasmodics can also be considered as treatment options, although their indications have not yet been established [31].
SCAD is known as diverticula-associated colitis and is diagnosed when there is chronic inflammation in an area of colonic diverticulosis without involvement of the diverticular orifice. The estimated prevalence of SCAD in diverticulosis is 1% [33,34]. Various clinical symptoms, including bleeding, diarrhea, and abdominal pain, can occur simultaneously. Endoscopy can reveal erythema, friability, and erosion, and computed tomography (CT) can reveal inflammation around the diverticula. Typically, inflammation spares the rectum and ascending colon [34]. The evidence for treatment is limited; however, antibiotics such as ciprofloxacin and metronidazole with mesalamine or oral steroids can be considered [35,36].
Aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) directly injure the colonic mucosa and inhibit prostaglandin synthesis. They increase intestinal permeability and reduce barrier function. Therefore, diverticular disease develops due to the influx of gut microbiota and toxins into the colon [37,38]. In addition, these drugs can aggravate diverticular bleeding owing to mucosal ulceration and decreased platelet function [39].
The association between aspirin or NSAIDs and diverticular disease has been demonstrated in a longitudinal study [40]. This large prospective cohort study in the United States included 47,210 males from the Health Professionals Follow-up Study who worked in the medical field in 1986 and were aged 40 years to 75 years. Data on aspirin and NSAIDs use were evaluated every 2 years since 1986, and the history of diverticular disease was collected every 2 years since 1990. A total of 939 diverticulitis and 256 diverticular bleeding cases were reported during 22 years of follow-up. After adjusting for other risk factors of diverticular disease, participants who took aspirin at least two times per week showed a significantly higher risk of diverticulitis than those in the control group (hazard ratio [HR], 1.25; 95% confidence interval [CI], 1.05–1.47), and the HR for diverticular bleeding was 1.70 (95% CI, 1.20–2.39). Another meta-analysis of 11 studies on NSAIDs indicated an association with diverticular perforation (odds ratio [OR], 3.4) and 12 studies indicated an association with diverticular bleeding (OR, 2.6) [41].
Although many clinicians recommend diet restriction during the acute phase of diverticulitis, the evidence is scarce. Considering that food intake increases peristalsis and pressure on the intestine, a restricted diet could be beneficial. However, this is not always necessary in outpatient settings in the absence of fever or other severe symptoms. A clear diet can be attempted in these cases and advanced based on symptom improvement within 3 to 5 days [15,42].
Diet modification is the best management strategy for incidental asymptomatic diverticulosis, and fiber intake can reduce the risk of diverticular disease-associated hospitalization [43,44]. Mild cases of symptomatic uncomplicated acute diverticulitis can be treated with broad-spectrum antibiotics against anaerobic microorganisms in outpatient clinics for 7 to 10 days [45-48]. Regarding treatment duration, one study indicated no significant difference between the 4- and 7-day treatment courses [49].
Hospitalization with intravenous antibiotics and fluid treatment is required for patients with severe symptoms or for older adults. Approximately 75% of patients who are hospitalized with acute diverticulitis respond favorably to medical treatment [50,51]. Clinical guidelines recommend changing intravenous antibiotics to oral agents if patients show clinical improvement without fever for 24 to 48 hours and a decreased leukocyte count [52]. Antibiotics must target both aerobic and anaerobic microbes. The most used antibiotics in outpatient clinics are a combination of ciprofloxacin and metronidazole [53]. In patients who are hospitalized, a combination of intravenous ceftriaxone and metronidazole, piperacillin and tazobactam, or meropenem as a single agent, can be used for 7 to 10 days [54].
A recent multicenter randomized clinical trial of 623 patients with uncomplicated acute diverticulitis showed no significant difference in recurrence or complications for 1 year after the episode between patients treated with and without antibiotics [55]. A meta-analysis by Hjern et al. [56] also indicated no additional benefits of antibiotics for uncomplicated left-sided diverticulitis. Similarly, a recent meta-analysis revealed that conservative treatment without antibiotics reduced the number of hospitalization days in patients who were clinically stable with uncomplicated diverticulitis [57]. Considering the risks of antibiotic resistance, cost, and adverse events, more care should be taken regarding antibiotic treatment for uncomplicated diverticulitis based on these recent data. Therefore, recent practice guidelines recommend antibiotics for uncomplicated mild diverticulitis only in cases of American Society of Anesthesiologists class III or IV comorbidities, persistent symptoms longer than 5 days prior to presentation, and a C-reactive protein level of >14 mg/dL or a white blood cell level of >15,000 cells/L [15].
Patients with uncomplicated diverticulitis can be treated in an outpatient setting, unless they have significant comorbidities or are immunosuppressed [47,48]. According to a retrospective study, patients with mildly complicated diverticulitis (abscess <4 cm or pneumoperitoneum <2 cm) can also be safely treated in outpatient clinics with close follow-up [58]. However, another retrospective study revealed a 50% rehospitalization rate after discharge from the emergency department [59]. More studies on the indications for hospitalization in mildly complicated diverticulitis are needed.
CT is the first and most widely used diagnostic modality because it is noninvasive and useful for evaluating inflammation around the diverticulum and detecting complications [63]. CT has an estimated sensitivity and specificity of 98% and 99%, respectively [64]. CT can be helpful in differentiating diverticulitis from other diseases such as colon cancer, appendicitis, epiploic appendagitis, ischemic colitis, and inflammatory bowel disease [64].
As CT can show the presence of a diverticulum with bowel wall thickening, pericolic infiltration, enhancement, abscess, obstruction, and lymph node enlargement in diverticulitis, differentiating diverticulitis from malignancy is sometimes difficult [65]. Therefore, colonoscopy is recommended to exclude the possibility of malignancy after an episode of diverticulitis, particularly in patients with no history of colonoscopy [66]. According to one systematic review evaluating the clinical utility of colonoscopy after acute diverticulitis diagnosed by abdominal CT, colonoscopy 4 to 8 weeks after a diverticulitis episode detected 15 cases of colon cancer and 38 cases of advanced adenoma [67].
As inflation with gas can result in colonic perforation, colonoscopy should be avoided during the acute phase. Therefore, colonoscopy is usually recommended at least 6 weeks after recovery from acute diverticulitis [68].
Although colonoscopy is a useful modality for diagnosing and treating colonic diverticular bleeding, it is challenging because the bleeding ceases spontaneously in 75% of cases [23]. After spontaneous hemostasis, identifying and treating the stigmata associated with a recent hemorrhage is difficult. Studies that indicated the effectiveness of colonoscopy within 24 hours of admission failed to show an improvement in the rebleeding rate within 30 days [69-71]. A recent multicenter randomized controlled study reported no significant difference in the stigmata of recent bleeding and rebleeding within 30 days between early (24 hours) and elective (>24 hours) colonoscopy. Another study showed that elective colonoscopy could be performed except in cases of positive extravasation on CT [72]. Another difficulty associated with early colonoscopy is bowel preparation. Bowel preparation is essential for visualizing lesions; however, it is time-consuming.
Although it did not show significant efficacy in improving the rebleeding rate, early colonoscopy with proper bowel preparation could help detect the bleeding focus, which is usually suggested in practice [42].
The old concept that the risk of complications increases after one or two recurrences has changed. Recent evidence has shown that the risk does not increase despite multiple recurrences [73]. Moreover, most complications develop during the first or second episode, except for fistulas. Based on these results, a personalized approach considering the number of recurrences, severity, and patient condition has been suggested [73]. Surgical treatment should be reserved for patients with peritonitis or perforation [73].
Although diverticular bleeding is the most common cause of hematochezia in patients who are older (≥60 years), only 10% to 15% of patients with diverticulosis experience bleeding, and 3% to 5% of these cases are severe [74-77]. More than 50% of diverticular bleeding cases stop spontaneously and only 4.6% require endoscopic intervention [78]. Surgery is rarely required in such cases. The indications for surgery include the requirement for transfusion of more than six units within 24 hours [79], recurrent hemorrhage that is refractory or not indicated for nonsurgical treatment, or hemodynamic instability [80]. The mortality rate of emergency surgery for uncontrolled bleeding is reportedly 10% to 20% [81,82].
Segmental resection should be considered only in confirmed bleeding foci, but the rebleeding rate is up to 14% [83]. Thus, a postoperative colonoscopy is needed to detect the bleeding focus and the presence of diverticulosis, and right or left colectomy should be performed. In cases of refractory bleeding from an undetectable focus, subtotal colectomy is required, with a high risk of complications and mortality [79].
Traditionally, diverticulitis has been considered to develop due to obstruction of the diverticulum opening, fecal stasis, and bacterial overgrowth [84]. However, the concept of diverticular disease as a chronic inflammatory disease has recently emerged [85,86]. Microscopic low-grade inflammation around the diverticulum without evidence of gross inflammation, an association between the degree of inflammation and symptom severity, and increased proinflammatory cytokines such as tumor necrosis factor-α could indicate that chronic inflammation is critical in the development of diverticular disease [87]. In addition, changes in gut microbiota, visceral hypersensitivity, and abnormal bowel motility interact in a complex manner to cause diverticular disease [1]. Based on this understanding of pathogenesis, studies on the efficacy of 5-aminosalicylates (5-ASAs), rifaximin, and probiotics in preventing diverticulitis recurrence and improving chronic inflammation have been reported [88-90].
A high-fiber diet appears to reduce the long-term risk of diverticulitis recurrence [91,92], but a recent systematic literature review reported limited evidence [93]. Although the importance of dietary fiber has waned recently, it is still recommended for its potential preventive benefits [91]. It is reasonable to recommend a high-fiber diet because it reduces the risk of symptomatic diverticular disease, including diverticulitis and bleeding [44]. It also has favorable effects on various chronic diseases, such as cardiovascular disease, obesity, and diabetes [1].
Recently, other medications such as rifaximin, 5-ASAs, and probiotics have been suggested to prevent diverticulitis. Rifaximin (a nonabsorbable antibiotic) significantly reduced the symptoms of diverticulitis at 1 year [32]; however, a recent study failed to confirm the role of rifaximin in secondary prevention and its proper dosing [94].
In the case of mesalamine, there are conflicting data, with only one positive result compared with placebo [95-97]. A 2017 Cochrane review of seven randomized controlled trials found no significant differences in diverticulitis recurrence. However, the data quality was very low and significantly heterogeneous [98]. Although some studies have shown a tendency for improved symptoms and reduced recurrence [99], there has been insufficient evidence to recommend probiotics in the prevention of diverticulitis [100]. In addition to these medications for secondary prevention, general recommendations for the prevention of diverticular disease, especially diverticulitis, are summarized in Table 1, although the evidence is limited [101].
In addition to traditional concepts, chronic low-grade inflammation, alterations in the gut microbiota, visceral hypersensitivity, and abnormal intestinal motility have recently emerged as important factors in the pathogenesis of diverticular disease. Although guidelines based on recent clinical studies provide recommendations regarding the diagnosis, management, and prevention of diverticular diseases, controversies remain regarding dietary modifications, prophylactic medications, surgical indications, and the use of antibiotics for uncomplicated diverticulitis. Further studies are required to confirm these issues based on routine practice.
References
1. Wan D, Krisko T. Diverticulosis, diverticulitis, and diverticular bleeding. Clin Geriatr Med. 2021; 37:141–54.
2. Ma W, Nguyen LH, Song M, Jovani M, Liu PH, Cao Y, et al. Intake of dietary fiber, fruits, and vegetables and risk of diverticulitis. Am J Gastroenterol. 2019; 114:1531–8.
3. Strate LL, Keeley BR, Cao Y, Wu K, Giovannucci EL, Chan AT. Western dietary pattern increases, and prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology. 2017; 152:1023–30.
4. Cao Y, Strate LL, Keeley BR, Tam I, Wu K, Giovannucci EL, et al. Meat intake and risk of diverticulitis among men. Gut. 2018; 67:466–72.
5. Song JH, Kim YS, Lee JH, Ok KS, Ryu SH, Lee JH, et al. Clinical characteristics of colonic diverticulosis in Korea: a prospective study. Korean J Intern Med. 2010; 25:140–6.
6. Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol. 2013; 11:1609–13.
7. Niikura R, Nagata N, Shimbo T, Aoki T, Yamada A, Hirata Y, et al. Natural history of bleeding risk in colonic diverticulosis patients: a long-term colonoscopy-based cohort study. Aliment Pharmacol Ther. 2015; 41:888–94.
8. Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000; 342:78–82.
9. Zullo A, Gatta L, Vassallo R, Francesco V, Manta R, Monica F, et al. Paradigm shift: the Copernican revolution in diverticular disease. Ann Gastroenterol. 2019; 32:541–53.
10. Touzios JG, Dozois EJ. Diverticulosis and acute diverticulitis. Gastroenterol Clin North Am. 2009; 38:513–25.
11. Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018; 12:125–32.
12. Bharucha AE, Parthasarathy G, Ditah I, Fletcher JG, Ewelukwa O, Pendlimari R, et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol. 2015; 110:1589–96.
13. Eckmann JD, Shaukat A. Updates in the understanding and management of diverticular disease. Curr Opin Gastroenterol. 2022; 38:48–54.
14. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association institute guideline on the management of acute diverticulitis. Gastroenterology. 2015; 149:1944–9.
15. Peery AF, Shaukat A, Strate LL. AGA clinical practice update on medical management of colonic diverticulitis: expert review. Gastroenterology. 2021; 160:906–11.
16. Eberhardt F, Crichton M, Dahl C, Nucera R, Jenkins J, Marx W, et al. Role of dietary fibre in older adults with asymptomatic (AS) or symptomatic uncomplicated diverticular disease (SUDD): systematic review and meta-analysis. Maturitas. 2019; 130:57–67.
17. Magidson PD, Martinez JP. Abdominal pain in the geriatric patient. Emerg Med Clin North Am. 2016; 34:559–74.
18. Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019; 156:254–72.
19. McGuire HH, Haynes BW. Massive hemorrhage for diverticulosis of the colon: guidelines for therapy based on bleeding patterns observed in fifty cases. Ann Surg. 1972; 175:847–55.
20. Meyers MA, Alonso DR, Gray GF, Baer JW. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology. 1976; 71:577–83.
21. Casarella WJ, Kanter IE, Seaman WB. Right-sided colonic diverticula as a cause of acute rectal hemorrhage. N Engl J Med. 1972; 286:450–3.
22. Wong SK, Ho YH, Leong AP, Seow-Choen F. Clinical behavior of complicated right-sided and left-sided diverticulosis. Dis Colon Rectum. 1997; 40:344–8.
23. McGuire HH. Bleeding colonic diverticula. A reappraisal of natural history and management. Ann Surg. 1994; 220:653–6.
24. Smoot RL, Gostout CJ, Rajan E, Pardi DS, Schleck CD, Harmsen WS, et al. Is early colonoscopy after admission for acute diverticular bleeding needed? Am J Gastroenterol. 2003; 98:1996–9.
25. Tursi A, Elisei W, Franceschi M, Picchio M, Di Mario F, Brandimarte G. The prevalence of symptomatic uncomplicated diverticular disease could be lower than expected: a single-center colonoscopy-based cohort study. Eur J Gastroenterol Hepatol. 2021; 33(1 Suppl):e478–83.
26. Bassotti G, Sietchiping-Nzepa F, De Roberto G, Chistolini F, Morelli A. Colonic regular contractile frequency patterns in irritable bowel syndrome: the 'spastic colon' revisited. Eur J Gastroenterol Hepatol. 2004; 16:613–7.
27. Bassotti G, Battaglia E, Bellone G, Dughera L, Fisogni S, Zambelli C, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol. 2005; 58:973–7.
28. Simpson J, Sundler F, Humes DJ, Jenkins D, Scholefield JH, Spiller RC. Post inflammatory damage to the enteric nervous system in diverticular disease and its relationship to symptoms. Neurogastroenterol Motil. 2009; 21:847–e58.
29. Barbaro MR, Cremon C, Fuschi D, Scaioli E, Veneziano A, Marasco G, et al. Nerve fiber overgrowth in patients with symptomatic diverticular disease. Neurogastroenterol Motil. 2019; 31:e13575.
30. Kvasnovsky CL, Leong LE, Choo JM, Abell GC, Papagrigoriadis S, Bruce KD, et al. Clinical and symptom scores are significantly correlated with fecal microbiota features in patients with symptomatic uncomplicated diverticular disease: a pilot study. Eur J Gastroenterol Hepatol. 2018; 30:107–12.
31. Tursi A, Papa A, Danese S. Review article: the pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment Pharmacol Ther. 2015; 42:664–84.
32. Bianchi M, Festa V, Moretti A, Ciaco A, Mangone M, Tornatore V, et al. Meta-analysis: long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment Pharmacol Ther. 2011; 33:902–10.
33. Mann NS, Hoda KK. Segmental colitis associated with diverticulosis: systematic evaluation of 486 cases with meta-analysis. Hepatogastroenterology. 2012; 59:2119–21.
34. Tursi A, Elisei W, Brandimarte G, Giorgetti GM, Lecca PG, Di Cesare L, et al. The endoscopic spectrum of segmental colitis associated with diverticulosis. Colorectal Dis. 2010; 12:464–70.
35. Schembri J, Bonello J, Christodoulou DK, Katsanos KH, Ellul P. Segmental colitis associated with diverticulosis: is it the coexistence of colonic diverticulosis and inflammatory bowel disease? Ann Gastroenterol. 2017; 30:257–61.
36. Rampton DS. Diverticular colitis: diagnosis and management. Colorectal Dis. 2001; 3:149–53.
37. Morris CR, Harvey IM, Stebbings WS, Speakman CT, Kennedy HJ, Hart AR. Epidemiology of perforated colonic diverticular disease. Postgrad Med J. 2002; 78:654–8.
38. Lanas A, Sopeña F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am. 2009; 38:333–52.
39. Foutch PG. Diverticular bleeding: are nonsteroidal anti-inflammatory drugs risk factors for hemorrhage and can colonoscopy predict outcome for patients? Am J Gastroenterol. 1995; 90:1779–84.
40. Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011; 140:1427–33.
41. Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteriodal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis. 2014; 16:O189–96.
42. Nagata N, Ishii N, Manabe N, Tomizawa K, Urita Y, Funabiki T, et al. Guidelines for colonic diverticular bleeding and colonic diverticulitis: Japan Gastroenterological Association. Digestion. 2019; 99(Suppl 1):1–26.
43. Crowe FL, Appleby PN, Allen NE, Key TJ. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ. 2011; 343:d4131.
44. Crowe FL, Balkwill A, Cairns BJ, Appleby PN, Green J, Reeves GK, et al. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014; 63:1450–6.
45. Sánchez-Velázquez P, Grande L, Pera M. Outpatient treatment of uncomplicated diverticulitis: a systematic review. Eur J Gastroenterol Hepatol. 2016; 28:622–7.
46. Balasubramanian I, Fleming C, Mohan HM, Schmidt K, Haglind E, Winter DC. Out-patient management of mild or uncomplicated diverticulitis: a systematic review. Dig Surg. 2017; 34:151–60.
47. van Dijk ST, Bos K, de Boer MG, Draaisma WA, van Enst WA, Felt RJ, et al. A systematic review and meta-analysis of outpatient treatment for acute diverticulitis. Int J Colorectal Dis. 2018; 33:505–12.
48. Cirocchi R, Randolph JJ, Binda GA, Gioia S, Henry BM, Tomaszewski KA, et al. Is the outpatient management of acute diverticulitis safe and effective? A systematic review and meta-analysis. Tech Coloproctol. 2019; 23:87–100.
49. Schug-Pass C, Geers P, Hügel O, Lippert H, Köckerling F. Prospective randomized trial comparing short-term antibiotic therapy versus standard therapy for acute uncomplicated sigmoid diverticulitis. Int J Colorectal Dis. 2010; 25:751–9.
50. Jacobs DO. Clinical practice. Diverticulitis. N Engl J Med. 2007; 357:2057–66.
51. Young-Fadok TM. Diverticulitis. N Engl J Med. 2018; 379:1635–42.
52. World Gastroenterology Organisation (WGO). WGO practice guideline [Internet]. Milwaukee: WGO; 2006 [updated 2007; cited 2024 May 17]. https://www.worldgastroenterology.org/guidelines/diverticular-disease/diverticular-disease-english.
53. Dichman ML, Rosenstock SJ, Shabanzadeh DM. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2022; 6:CD009092.
54. Weizman AV, Nguyen GC. Diverticular disease: epidemiology and management. Can J Gastroenterol. 2011; 25:385–9.
55. Chabok A, Påhlman L, Hjern F, Haapaniemi S, Smedh K; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012; 99:532–9.
56. Hjern F, Josephson T, Altman D, Holmström B, Mellgren A, Pollack J, et al. Conservative treatment of acute colonic diverticulitis: are antibiotics always mandatory? Scand J Gastroenterol. 2007; 42:41–7.
57. Mohamedahmed AY, Zaman S, Das N, Kakaniaris G, Vakis S, Eccersley J, et al. Systematic review and meta-analysis of the management of acute uncomplicated diverticulitis: time to change traditional practice. Int J Colorectal Dis. 2024; 39:47.
58. Joliat GR, Emery J, Demartines N, Hübner M, Yersin B, Hahnloser D. Antibiotic treatment for uncomplicated and mild complicated diverticulitis: outpatient treatment for everyone. Int J Colorectal Dis. 2017; 32:1313–9.
59. Sirany AE, Gaertner WB, Madoff RD, Kwaan MR. Diverticulitis diagnosed in the emergency room: is it safe to discharge home? J Am Coll Surg. 2017; 225:21–5.
60. Hall J, Hardiman K, Lee S, Lightner A, Stocchi L, Paquette IM, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of left-sided colonic diverticulitis. Dis Colon Rectum. 2020; 63:728–47.
61. Qaseem A, Etxeandia-Ikobaltzeta I, Lin JS, Fitterman N, Shamliyan T, Wilt TJ, et al. Diagnosis and management of acute left-sided colonic diverticulitis: a clinical guideline from the American College of Physicians. Ann Intern Med. 2022; 175:399–415.
62. Sartelli M, Catena F, Ansaloni L, Coccolini F, Griffiths EA, Abu-Zidan FM, et al. WSES Guidelines for the management of acute left sided colonic diverticulitis in the emergency setting. World J Emerg Surg. 2016; 11:37.
63. Birnbaum BA, Balthazar EJ. CT of appendicitis and diverticulitis. Radiol Clin North Am. 1994; 32:885–98.
64. Hawkins AT, Wise PE, Chan T, Lee JT, Glyn T, Wood V, et al. Diverticulitis: an update from the age old paradigm. Curr Probl Surg. 2020; 57:100862.
65. Shen SH, Chen JD, Tiu CM, Chou YH, Chiang JH, Chang CY, et al. Differentiating colonic diverticulitis from colon cancer: the value of computed tomography in the emergency setting. J Chin Med Assoc. 2005; 68:411–8.
66. Hale WB; National Diverticulitis Study Group. Colonoscopy in the diagnosis and management of diverticular disease. J Clin Gastroenterol. 2008; 42:1142–4.
67. Daniels L, Unlü C, de Wijkerslooth TR, Dekker E, Boermeester MA. Routine colonoscopy after left-sided acute uncomplicated diverticulitis: a systematic review. Gastrointest Endosc. 2014; 79:378–89.
68. Almy TP, Howell DA. Medical progress. Diverticular disease of the colon. N Engl J Med. 1980; 302:324–31.
69. Kouanda AM, Somsouk M, Sewell JL, Day LW. Urgent colonoscopy in patients with lower GI bleeding: a systematic review and meta-analysis. Gastrointest Endosc. 2017; 86:107–17.
70. Laine L, Shah A. Randomized trial of urgent vs. elective colonoscopy in patients hospitalized with lower GI bleeding. Am J Gastroenterol. 2010; 105:2636–41.
71. Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005; 100:2395–402.
72. Ochi M, Kamoshida T, Hamano Y, Ohkawara A, Ohkawara H, Kakinoki N, et al. Early colonoscopy and urgent contrast enhanced computed tomography for colonic diverticular bleeding reduces risk of rebleeding. World J Clin Cases. 2021; 9:2446–57.
73. Rafferty J, Shellito P, Hyman NH, Buie WD; Standards Committee of American Society of Colon and Rectal Surgeons. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006; 49:939–44.
74. Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology. 1988; 95:1569–74.
75. Niikura R, Nagata N, Yamada A, Akiyama J, Shimbo T, Uemura N. Recurrence of colonic diverticular bleeding and associated risk factors. Colorectal Dis. 2012; 14:302–5.
76. Okamoto T, Watabe H, Yamada A, Hirata Y, Yoshida H, Koike K. The association between arteriosclerosis related diseases and diverticular bleeding. Int J Colorectal Dis. 2012; 27:1161–6.
77. Nishikawa H, Maruo T, Tsumura T, Sekikawa A, Kanesaka T, Osaki Y. Risk factors associated with recurrent hemorrhage after the initial improvement of colonic diverticular bleeding. Acta Gastroenterol Belg. 2013; 76:20–4.
78. Ron-Tal Fisher O, Gralnek IM, Eisen GM, Williams JL, Holub JL. Endoscopic hemostasis is rarely used for hematochezia: a population-based study from the Clinical Outcomes Research Initiative National Endoscopic Database. Gastrointest Endosc. 2014; 79:317–25.
79. Hall J, Hammerich K, Roberts P. New paradigms in the management of diverticular disease. Curr Probl Surg. 2010; 47:680–735.
80. Wilkins T, Baird C, Pearson AN, Schade RR. Diverticular bleeding. Am Fam Physician. 2009; 80:977–83.
81. Chen CY, Wu CC, Jao SW, Pai L, Hsiao CW. Colonic diverticular bleeding with comorbid diseases may need elective colectomy. J Gastrointest Surg. 2009; 13:516–20.
82. Britt LG, Warren L, Moore OF. Selective management of lower gastrointestinal bleeding. Am Surg. 1983; 49:121–5.
83. Parkes BM, Obeid FN, Sorensen VJ, Horst HM, Fath JJ. The management of massive lower gastrointestinal bleeding. Am Surg. 1993; 59:676–8.
84. Tursi A, Scarpignato C, Strate LL, Lanas A, Kruis W, Lahat A, et al. Colonic diverticular disease. Nat Rev Dis Primers. 2020; 6:20.
85. Lahat A, Necula D, Yavzori M, Picard O, Halperin S, Eliakim R, et al. Prolonged recurrent abdominal pain is associated with ongoing underlying mucosal inflammation in patients who had an episode of acute complicated diverticulitis. J Clin Gastroenterol. 2019; 53:e178–85.
86. Tursi A, Elisei W, Giorgetti GM, Inchingolo CD, Nenna R, Picchio M, et al. Detection of endoscopic and histological inflammation after an attack of colonic diverticulitis is associated with higher diverticulitis recurrence. J Gastrointestin Liver Dis. 2013; 22:13–9.
87. Humes DJ, Simpson J, Smith J, Sutton P, Zaitoun A, Bush D, et al. Visceral hypersensitivity in symptomatic diverticular disease and the role of neuropeptides and low grade inflammation. Neurogastroenterol Motil. 2012; 24:318–e163.
88. Barbara G, Cremon C, Barbaro MR, Bellacosa L, Stanghellini V. Treatment of diverticular disease with aminosalicylates: the evidence. J Clin Gastroenterol. 2016; 50(Suppl 1):S60–3.
89. Scarpignato C, Bertelé A, Tursi A. Probiotics for the treatment of symptomatic uncomplicated diverticular disease: rationale and current evidence. J Clin Gastroenterol. 2016; 50(Suppl 1):S70–3.
90. Stallinger S, Eller N, Högenauer C. Non-interventional study evaluating efficacy and tolerability of rifaximin for treatment of uncomplicated diverticular disease. Wien Klin Wochenschr. 2014; 126:9–14.
91. de Korte N, Kuyvenhoven JP, van der Peet DL, Felt-Bersma RJ, Cuesta MA, Stockmann HB. Mild colonic diverticulitis can be treated without antibiotics. A case-control study. Colorectal Dis. 2012; 14:325–30.
92. Stollman NH, Raskin JB; Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Diagnosis and management of diverticular disease of the colon in adults. Am J Gastroenterol. 1999; 94:3110–21.
93. Dahl C, Crichton M, Jenkins J, Nucera R, Mahoney S, Marx W, et al. Evidence for dietary fibre modification in the recovery and prevention of reoccurrence of acute, uncomplicated diverticulitis: a systematic literature review. Nutrients. 2018; 10:137.
94. Moniuszko A, Rydzewska G. The effect of cyclic rifaximin therapy on symptoms of diverticular disease from the perspective of the gastroenterology outpatient clinic: a "real-life" study. Prz Gastroenterol. 2017; 12:145–51.
95. Khan RM, Ali B, Hajibandeh S, Hajibandeh S. Effect of mesalazine on recurrence of diverticulitis in patients with symptomatic uncomplicated diverticular disease: a meta-analysis with trial sequential analysis of randomized controlled trials. Colorectal Dis. 2018; 20:469–78.
96. Iannone A, Ruospo M, Wong G, Barone M, Principi M, Di Leo A, et al. Mesalazine for people with diverticular disease: a systematic review of randomized controlled trials. Can J Gastroenterol Hepatol. 2018; 2018:5437135.
97. Picchio M, Elisei W, Brandimarte G, Di Mario F, Malfertheiner P, Scarpignato C, et al. Mesalazine for the treatment of symptomatic uncomplicated diverticular disease of the colon and for primary prevention of diverticulitis: a systematic review of randomized clinical trials. J Clin Gastroenterol. 2016; 50(Suppl 1):S64–9.
98. Carter F, Alsayb M, Marshall JK, Yuan Y. Mesalamine (5-ASA) for the prevention of recurrent diverticulitis. Cochrane Database Syst Rev. 2017; 10:CD009839.
99. Banasiewicz T, Francuzik W, Bobkiewicz A, Krokowicz Ł, Borejsza-Wysocki M, Paszkowski J, et al. The influence of rifaximin on diverticulitis rate and quality of life in patients with diverticulosis. Pol Przegl Chir. 2017; 89:22–31.
100. Lahner E, Bellisario C, Hassan C, Zullo A, Esposito G, Annibale B. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis. 2016; 25:79–86.
101. Böhm SK, Kruis W. Lifestyle and other risk factors for diverticulitis. Minerva Gastroenterol Dietol. 2017; 63:110–8.
Table 1.
Suggested recommendations for the prevention of diverticular disease




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download