Introduction
Study design
Table 1.
Inclusion and exclusion criteria
Results
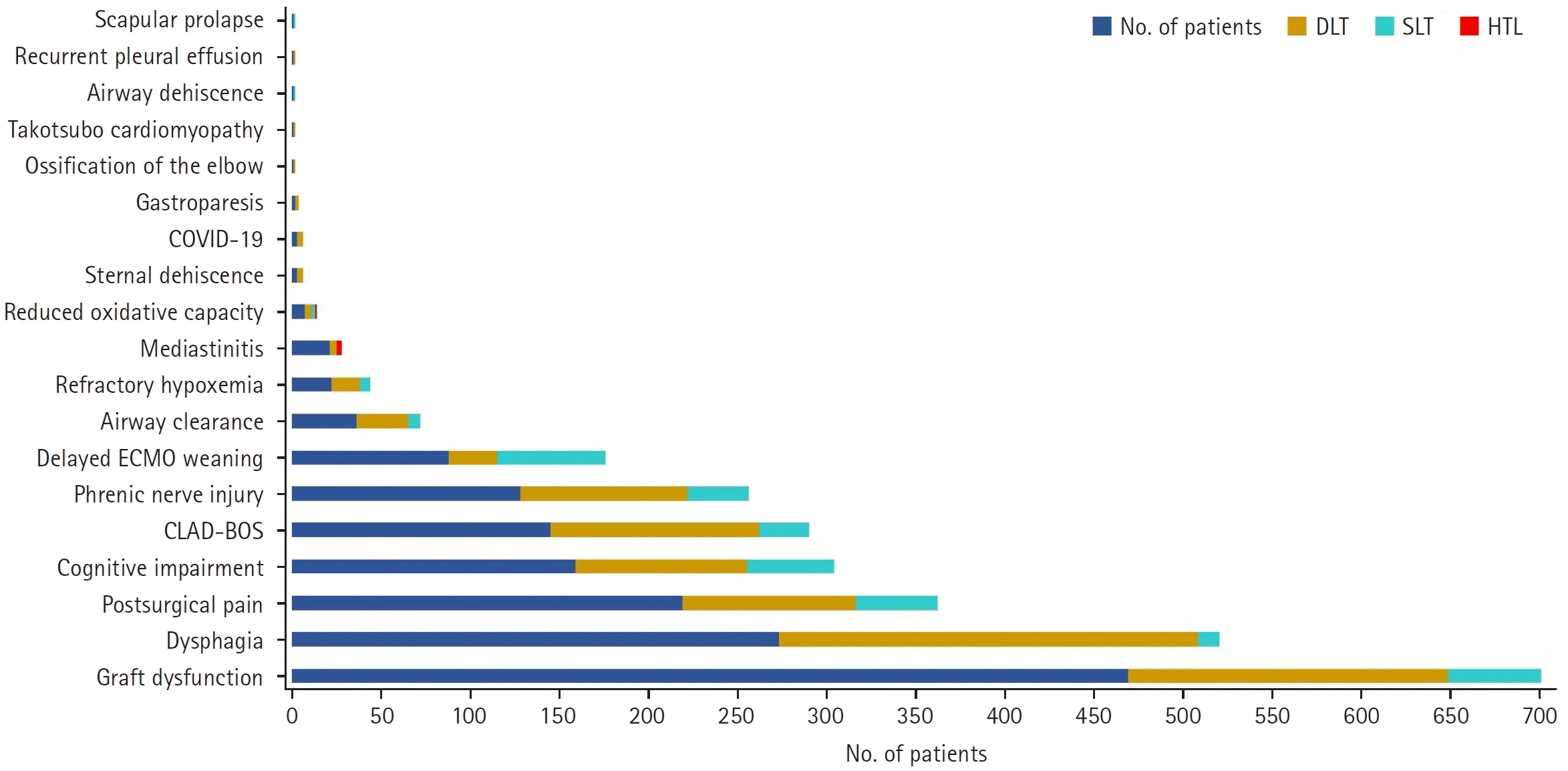 | Fig. 2.Conditions of rehabilitative interest and number of patients. DLT, double lung transplant; SLT, single lung transplant; HLT, heart-lung transplant; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; CLAD-BOS, chronic lung allograft dysfunction-bronchiolitis obliterans syndrome. |
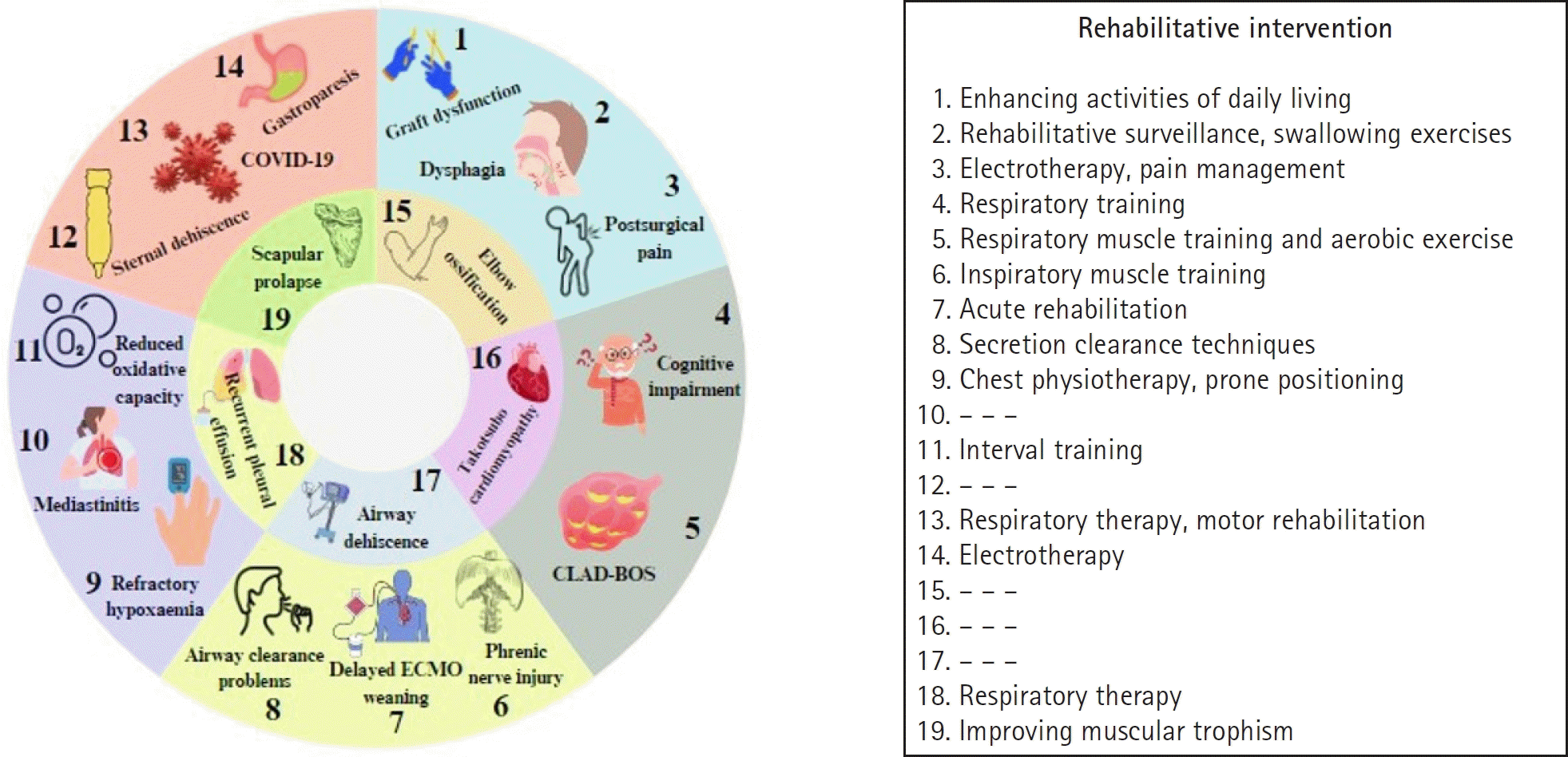 | Fig. 3.Conditions of rehabilitative interest in lung transplantation. COVID-19, coronavirus disease 2019; CLAD-BOS, chronic lung allograft dysfunction-bronchiolitis obliterans syndrome; ECMO, extracorporeal membrane oxygenation. |
Table 2.
| Study | Year | Country | Study design | Patients | No. of males (%) |
|---|---|---|---|---|---|
| Yu et al. [48] | 2023 | Canada | Retrospective | 140 patients with a mean age of 47.2±13.6 years underwent solid organ transplantation; of these, 64 were LT (61 DLT, 3 SLT). Patients were monitored for opioid consumption. Pain was evaluated at pretransplant visit, 15±11 days posttransplant, 29±11 days after the first follow-up, and 210±636 days after the first follow-up. Patients were offered to enroll in a physiotherapy consultation to manage postsurgical pain | 80 (57) |
| Wildgaard et al. [49] | 2010 | Denmark | Cross-sectional (survey) | 79 patients (46% DLT) with a mean age of 50 years (IQR 21–69 years) were invited to complete a questionnaire and describe their pain perception at a mean follow-up of 39 months after surgery | 36 (46) |
| Li et al. [50] | 2023 | China | Comparative | 88 LT recipients were allocated into two groups; 41 aged 54 years (IQR 50–64 years) were immediately weaned from VV-ECMO after LT, and 47 aged 60 years (IQR 49.5–64.5 years) had delayed weaning | 78 (89) |
| Dallal-York et al. [51] | 2022 | USA | Retrospective | 205 patients with a mean age of 58.6±13.7 years undergoing LT executed posttransplant VFSE to assess swallowing safety. 170 patients aged 59.8±12.4 years out of 205 underwent pretransplant VFSE | 104 (51) |
| Black et al. [52] | 2019 | Australia | Cross-sectional | 68 patients with a mean age of 48.3±13 years were referred to speech pathology consultation; 67 out of 68 underwent evaluation of voice | 40 (59) |
| Hernández-Hernández et al. [53] | 2022 | Spain | Prospective | 127 LT recipients aged 59 years (IQR 53–62 years) were assessed for phrenic nerve injury by diaphragm ultrasound examination and phrenic nerve conduction. Diaphragm ultrasound and phrenic nerve conduction studies were performed 3 days (IQR 2–6 days) days and 18 days (IQR 8–21 days) after LT | 89 (70) |
| Cohen et al. [54] | 2014 | USA | Retrospective | 56 patients, 14 LT candidates with a median age of 60 years (IQR 56–65 years), and 42 LT recipients aged 60 years (IQR 57–64 years) were screened to examine cognition using the MoCA | 24 (43) |
| Tomasi et al. [55] | 2022 | Germany | Prospective | 24 patients were allocated into two groups: 14 with a median age of 55 years (IQR 20.5–59 years) in the POCD group and 10 aged 59 years (45.5–65 years) in the non-POCD group to evaluate the postoperative cognitive function using a battery of neuropsychological tests (VVLT, SCWT, CST) | 8 (33) |
| Cao et al. [56] | 2024 | China | Cross-sectional (survey) | 79 patients with a mean age of 51.6±14.5 years returned a questionnaire investigating POCD by using the MoCA at four time points: 8 days, 1 month, 3 months, and 6 months after surgery | 63 (80) |
| Armstrong et al. [57] | 2016 | USA | Retrospective | 243 LT recipients with a median age of 56 years (IQR 42–62 years) were assessed to compare the long-term functional outcomes of those who developed grade 3 PGD with those who did not | 119 (49) |
| Kolaitis et al. [58] | 2021 | USA | Prospective | 226 LT recipients with a mean age of 55.7±12.6 years were assessed to test whether PGD was associated with increased disability (LT-VLA scale), depression (GDS), and poorer HRQL (SF12-PCS and SF12-MCS) | 125 (55) |
| Riera et al. [59] | 2017 | Spain | Prospective | 22 LT recipients with a median age of 58 years (IQR 53–62 years) out of 131 (16.8%) received prone positioning because of postoperative refractory hypoxemia with the need for a FiO2 >0.7 for a PaO2 >80 mmHg. Prone positioning was stopped when PaO2/FiO2 >150 mmHg, with a FiO2 <0.6, and when improvement was maintained for at least 4 hours in the supine position | 15 (68) |
| van Den Berg et al. [60] | 2000 | Netherlands | Cross-sectional | 116 LT recipients were evaluated postoperatively to define the incidence of CLAD-BOS. Patients were allocated into two groups: (1) LT recipients not developing CLAD-BOS were 64 with a mean age of 43±12 years, and (2) LT recipients developing CLAD-BOS were 52 with a mean age of 42±13 years | 35 (41) |
| Vermeulen et al. [61] | 2004 | Netherlands | Observational | The study cohort comprised 29 LT recipients with a mean age of 45 years (IQR 21–52 years) who completed the follow-ups and returned the questionnaires for assessing HRQL | 18 (62) |
| Abid et al. [62] | 2003 | UK | Retrospective | 21 patients with a mean age of 42.8±15.1 years developed mediastinitis (presence of pus or bacterial growth, or both, in mediastinal tissue either with or without sternal instability) posttransplant, of these, 3 were HLT and 4 DLT | 18 (86) |
| Xu et al. [63] | 2018 | China | Case report | A 44-year-old woman LT recipient suffered failed weaning from MV on POD 3 and was evaluated with diaphragm electromyography. The TwPdi measurements under magnetic stimulation of the phrenic nerves showed a bilateral PNCT of 13 milliseconds and bilateral diaphragmatic CMAPs of 0.508 mV | 0 (0) |
| Munin et al. [64] | 1995 | USA | Case report | A 37-year-old woman received a DLT and, on POD 18, presented with reduced ROM in both elbows. X-ray examination showed bilateral heterotopic ossification along the posterior humerus and ulna. The patient underwent right elbow osteotomy resection of the heterotopic ossification and anterior ulnar nerve transposition | 0 (0) |
| Keller et al. [65] | 2020 | USA | Case report | A 68-year-old woman underwent DLT and, on POD 9, tested positive for COVID-19 after having developed worsening hypoxemia with extensive pulmonary edema consistent with grade 3 PGD. On POD 14, the test was negative, and on POD 30, the patient underwent a tracheostomy | 0 (0) |
| Duclos et al. [66] | 2018 | France | Case report | A 50-year-old man underwent DLT and had cardiac arrest during induction; transesophageal echocardiography showed global hyperkinetic left ventricular function with supranormal cardiac output, no regional hypokinesia of the left ventricle, and no obstruction or dilatation of the right ventricle | 1 (100) |
| Backer et al. [67] | 2020 | USA | Case report | A 66-year-old man SLT recipient with comorbidities developed a fungal empyema and partial dehiscence of the right anastomosis; in addition, he had exertional dyspnea and required oxygen supplementation (2 L/min). Surgery to treat airway dehiscence was not considered a viable option because of the complicated postoperative course, infected pleural space, and physical deconditioning. The dehiscence was then treated by applying thermal energy around the edge to promote neoepithelialization | 1 (100) |
| Panchabhai et al. [68] | 2015 | USA | Case report | A 29-year-old woman DLT recipient underwent retransplantation because of CLAD-BOS 6 years after the first LT. The patient was weaned from MV and started physical rehabilitation. After 2 weeks, the patient presented with hypoxemia and right pleural effusion (600 mL). Because of persistent pleural fluid accumulation tube thoracostomy was placed with the evacuation of 1,200 mL of milky white fluid. Pleural fluid cultures grew Candida glabarata, Burkholderia cepacia, and Enterococcus fecium. Given the polymicrobial growth of the pleural fluid culture with organisms similar to the patient’s sinus culture and enteric sources, esophageal perforation was suspected and confirmed by an exploratory thoracotomy | 0 (0) |
| Chansakul et al. [69] | 2014 | USA | Case report | A 76-year-old woman SLT recipient, and 8 months after LT, the patient presented with shoulder pain which radiated down her back and she was unable to lift her arm overhead. Pain on more than 30° of forward flexion and 30° abduction of the right shoulder was present. CT scan showed an abnormal position of the right scapula, with the inferior angle of the scapula protruding into the right intrathoracic cavity | 0 (0) |
| Orsini et al. [70] | 2014 | France | Case series | 3 DLT recipients (mean age 47.7±9 years) out of 61 developed sternal dehiscence 2–3 months after surgery | 2 (67) |
| Weinkauf et al. [71] | 2005 | USA | Case series | Twp DLT recipients with a mean age of 40 years (IQR 26–54 years) developed postoperative gastroparesis (delayed passage of gastric contents into the intestine) and were treated with TENS at 18 months and 8 months after surgery, respectively | 1 (50) |
| Gergen et al. [72] | 2021 | USA | Case series | Two DLT recipients with a mean age of 59 years (IQR 56–62 years) presented with COVID-19; one patient was intubated, paralyzed, and proned, and on hospital day 19, she underwent tracheostomy. The other patient required 8 L/min oxygen supplementation and was treated with remdesivir and empiric cefepime | 0 (0) |
| Wang et al. [73] | 1999 | Australia | Case series | Seven patients (4 DLT, 2 SLT, 1 HLT) with a mean age of 37±4.3 years were evaluated at 12±8.2 months after LT to verify if the reduced oxidative capacity of peripheral skeletal muscle caused exercise limitation measuring mitochondrial oxidative phosphorylation, metabolic enzyme activity (oxidative and glycolytic), and fiber type proportion. Data were compared with seven healthy volunteers matched per age and sex | 3 (43) |
| Munro et al. [74] | 2008 | Australia | RCT | 36 LT recipients were allocated to two groups to compare the effects of a proactive vs. a reactive airway clearance regime using PEP therapy. In the proactive group, patients had a mean age of 45.1±3.2 years, while in the reactive was 47.5±3.6 years. The proactive strategy consisted of twice daily airway clearance with a PEP mask, while the reactive PEP therapy was performed only if patients had found four of six common clinical signs of chest infection (change in sputum production, increased cough, increased dyspnea, fever, radiographic changes indicative of pulmonary infection, positive sputum culture) | 18 (50) |
LT, lung transplant; DLT, double lung transplant; SLT, single lung transplant; IQR, interquartile range; VV-ECMO, venovenous extracorporeal membrane oxygenation; VFSE, videofluoroscopic swallowing exams; MoCA, Montreal Cognitive Assessment; POCD, postoperative cognitive dysfunction; VVLT, Visual Verbal Learning Test; SCWT, Stroop Color Word Test; CST, Concept Shifting Test; PGD, primary graft dysfunction; LT-VLA, Lung Transplant Valued Life Activities; GDS, Geriatric Depression Scale; HRQL, health-related quality of life; SF12-PCS, Short Form-12 Physical Component Score; SF12-MCS, Short Form-12 Mental Component Score; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen; CLAD-BOS, chronic lung allograft dysfunction-bronchiolitis obliterans syndrome; HLT, heart-lung transplant; MV, mechanical ventilation; TwPdi, twitch transdiaphragmatic pressure; PNCT, phrenic nerve conduction time; CMAPs, compound motor action potentials; mV, millivolt; POD, postoperative day; ROM, range of motion; COVID-19, coronavirus disease 2019; CT, computed tomography; TENS, transcutaneous electrical nerve stimulation; RCT, randomized controlled trial; PEP, positive expiratory pressure.
Table 3.
| No. | Condition | Main findings |
|---|---|---|
| 1 | Graft dysfunction | · 32% of patients had grade 3 PGD (presence of new parenchymal infiltrates in the lung allograft consistent with pulmonary edema and a PaO2/FiO2 <200 mmHg at 72 hours after LT). Differences in functional outcomes between patients with PGD+ and PGD– were not significant: Watts (% predicted peak) 51%±19% vs. 51%±15% (p=0.89), 6-MWD 485±93 m vs. 486±99 m (p=0.96) [57] |
| · 20% of patients developed grade 3 PGD. Pretransplant LT-VLA, GDS, SF12-PCS, and SF12-MCS were similar between participants with grade 3 PGD+ compared to those with PGD– (p>0.49). Improvements in LT-VLA, GDS, SF12-PCS, and SF12-MCS peaked 6 months after LT and remained relatively stable thereafter. In patients with grade 3 PGD, at 2-year follow-up, LT-VLA exceeded 2-fold the MCID, GDS exceeded 1-fold the MCID, SF12-PCS exceeded 2-fold the MCID, and SF12-MCS exceeded 1-fold the MCID [58] | ||
| 2 | Dysphagia | · Among the 205 patients who underwent postoperative VFSE, 20% demonstrated safe swallowing and 40% aspiration. In 170 patients who executed VFSE pre- and postoperatively, 83% demonstrated safe swallowing and 7% aspiration preoperatively; among aspirators, 50% could not eject aspirate material. Postoperatively, in the same cohort of 170 LT recipients, 16% demonstrated safe swallowing, and 45% aspiration; among aspirators, silent aspiration (accidentally inhaling something without noticing) was present in 47% [51] |
| · Among 68 patients referred to speech pathology consultation, 66 underwent bedside assessment; 88% presented with oropharyngeal dysphagia. Among patients who underwent voice assessment, 62% presented with mild to severe laryngeal dysfunction, 16% were diagnosed with vocal fold palsy or paresis, and 90% of them also presented with dysphagia [52] | ||
| 3 | Postsurgical pain | · At the last visit, the BPI average pain score decreased from 5.6±1.8 to 4.9±2.2 points. HADS passed from 16±8.7 to 15.1±8.1 points. PCS varied from 21.3±15 to 18.3±12.5 points. SPTS varied from 33.4±11.1 to 29.4±10.3 points, and SF-MPQ-2 varied from 5.1±2.2 to 4.1±2.4 points [48] |
| · 18% of participants reported persistent postsurgical pain; in 62% of them, NRS was >3 when physically active and walking. In 31% of patients, NRS was >5 when walking and 15% when physically active. 54% complained of pain during mild activities, 50% when rising from a chair or sitting down for >30 minutes or walking on stairs, and 46% while standing up for >30 minutes. In >30% of patients, pain was from more than one single body site, including the head, back, knee/hip [49] | ||
| 4 | Cognitive impairment | · The MoCA among LT candidates and recipients was 25±2 and 24±3, respectively. A mild impairment was present in 71% of LT candidates and 71% of LT recipients [54] |
| · Postoperative neurocognitive dysfunction was present in 58% of patients (14 out of 24). The CST was significantly impaired in the number of errors (p=0.006) in the POCD group. The time needed (p=0.002) and the number of errors (p=0.016) of the SCWT were significantly higher in the POCD group [55] | ||
| · Patients who participated in early postoperative rehabilitation had a lower risk of POCD (p<0.05) [56] | ||
| 5 | CLAD-BOS | · Among 116 LT recipients, 52 (45%) developed CLAD-BOS; in these patients, the NHP was higher (worse) at all follow-ups at 4,7,13,19,25,31,37,43,49 months, and differences were significant at 7,13,19,25,31 months (p<0.05, p<0.01) [60] |
| · The NHP score deteriorated over 18 months posttransplant, and differences in the Energy and Mobility domains at 3 and 18 months (0 vs. 24 and 0 vs. 22 points, respectively) were significant (p<0.001). To the same extent, the STAI score deteriorated, although differences were not significant (34.08±11.34 vs. 39.79±12.73 points); the ZUNG SDS also passed from 40.89±11.73 to 49.39±12.84 points (p<0.001) and the IWB from 12.21±2.55 to 9.92±3.34 points (p=0.001) [61] | ||
| 6 | Phrenic nerve injury | · Phrenic nerve injury was detected in 43% of patients; the lesion was bilateral in 7.1%, on the right side in 23.6%, and on the left in 12.6%. The incidence was doubled in DLT vs. SLT (50% vs. 24%) [53] |
| · Adjustments of pressure support ventilation from 12 cmH2O to 8 cmH2O were correlated with changes in EMGdi (9.8±0.71 μV vs. 17.1±1.28 μV). At 3 months, the left PNCT was 10 milliseconds, the left CMAPs were 0.970 mV, the right PNCT 11 milliseconds, and the right CMAPs were 0.837 mV. Decreased left CMAPs, prolonged left PNCT, and failure to induce right CMAPs and PNCT under bilateral magnetic stimulation were consistent with right phrenic nerve injury [63] | ||
| 7 | Delayed ECMO weaning | · Delayed weaning from VV-ECMO compared to immediate postoperative weaning was correlated with shorter hospital length of stay (31 vs. 46 days) and lower incidence of NIV (4.3% vs. 24.4%) and PGD (6.4% vs. 29.3%), longer ICU stay (92 vs. 88 days), longer duration of MV (44 vs. 27 hours), and higher mortality rates (10.6% vs. 7.3%) [50] |
| 8 | Airway clearance | · There was a significant improvement in FEV1 (72%±4% to 81%±4%, p<0.0001) and FVC (69%±3% to 81%±3%, p<0.0001) with no significant differences between groups. CXR scores improved in both groups (17.8±0.5 at 1 month to 19.8±0.5 at 3 months, p=0.002), but no difference existed between groups. Self-reported adherence was 84% in the proactive group and 100% in the reactive. 68% of patients in the proactive group and 72% in the reactive reported no secretions at 2 months [74] |
| 9 | Refractory hypoxemia | · In 15 patients (68.2%), prone positioning was implemented within the first 72 hours after surgery and was maintained for a median of 21 hours (IQR 14.2–24 hours). PaO2/FiO2 increased from 81 mmHg (IQR 71.5–104 mmHg) to 220 (IQR 160–288 mmHg) (p<0.001). The PaCO2 changed from 46 mmHg (IQR 38–54 mmHg) to 40.5 mmHg (IQR 38.2–45.5 mmHg) (p=0.01), and the pH from 7.36 (IQR 7.28–7.43) to 7.39 (IQR 7.34–7.47) (p<0.001) [59] |
| 10 | Mediastinitis | · Six deaths (28%) occurred, 33% of which were HLT and 50% DLT. Staphylococcus aureus contamination had a better prognosis (89% survived) than polymicrobial or fungal mediastinitis (33% survived) [62] |
| 11 | Reduced oxidative capacity | · LT recipients showed a lower peak work rate (88±10 vs. 218±30 W, p<0.005), VO2 peak (18.7±1.5 vs. 36.9±2.4 mL/kg/min, p<0.05), HR peak (137±6 vs. 177±5 bpm), and shorter exercise duration (5.4±0.6 vs. 9±1 minutes, p<0.05) compared to healthy controls. LT recipients exhibited a lower proportion of type I muscle fibers (24.9%±4.4% vs. 56.1%±2.4%, p<0.001). In resting skeletal muscle, lactate was higher (16.3±1 vs. 8.4±0.9 mmol/L, p<0.01), and ATP was lower (21.4±1.2 vs. 26±1.3 RLU, p<0.01) in LT recipients who also exhibited lower activity of the mitochondrial enzymes (p<0.005) [73] |
| 12 | Sternal dehiscence | · The STRATOS device stabilized sternal dehiscence; consolidation was obtained within 2 months in all three patients [70] |
| 13 | COVID-19 | · The patient was liberated from MV on POD 57 and prosecuted with physical therapy to counteract physical motor deconditioning [65] |
| · One patient was weaned from a ventilator and decannulated on hospital day 38 and discharged to a rehabilitation facility. The other patient, after a first hospitalization of 6 days, was readmitted and started broad-spectrum antibiotics and high-dose steroids; the patient did not wish to be intubated and expired on hospital day 3 [72] | ||
| 14 | Gastroparesis | · TENS was applied with two electrodes placed in the infrascapular region and in correspondence with T5-T10 vertebrae. In one case, treatment lasted 21 hours, while in the other 19 days, both patients experienced significant improvements after the first two sessions. The electrical stimulation consisted of a 20-mA current at a rate of 150 Hz delivered in a continuous sine wave pattern for 30 minutes in both cases. At 6-month (in one case) and 1-year (in the other case) follow-up, patients were free from symptoms and stopped promobility medication [71] |
| 15 | Ossification of the elbow | · After surgical release, the ROM in the right elbow improved from –60° to 75° to –10° to 125°. The left elbow was not treated because the patient moved to another area [64] |
| 16 | Takotsubo cardiomyopathy | · The patient was extubated 6 hours after surgery, and transthoracic echocardiogram performed on POD 10 found complete recovery of left ventricle function and wall motion abnormalities. The patient was discharged home on POD 40 [66] |
| 17 | Airway dehiscence | · The procedure was well-tolerated and free from postoperative complications; at 8 weeks, complete resolution of the dehiscence was obtained, and the patient was weaned from oxygen therapy, proceeding with physical rehabilitation [74] |
| 18 | Recurrent pleural effusion | · The patient had a complicated course, and esophagrams demonstrated a continued leak. Five months after retransplantation, the patient died of respiratory failure and debility [68] |
| 19 | Scapular prolapse | · Under mild sedation, the right scapula was reduced with axial traction along the medial border while passively forward flexing the right shoulder. The patient was instructed not to move the arm for 2 weeks and immobilized and then participated in a rehabilitation program to strengthen the periscapular musculature. At follow-up, the patient had full ROM and no pain [69] |
PGD, primary graft dysfunction; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; LT, lung transplant; 6-MWD, 6-minute walking distance; LT-VLA, Lung Transplant Valued Life Activities; GDS, Geriatric Depression Scale; SF12-PCS, Short Form-12 Physical Component Score; SF12-MCS, Short Form-12 Mental Component Score; MCID, minimal clinically important difference; VFSE, videofluoroscopic swallowing exams; BPI, Brief Pain Inventory; HADS, Hospital Anxiety and Depression Scale; PCS, Pain Catastrophizing Scale; SPTS, Sensitivity to Pain Traumatization Scale; SF-MPQ-2, Short McGill Pain Questionnaire-2; NRS, Numerical Rating Scale; MoCA, Montreal Cognitive Assessment; CST, Concept Shifting Test; POCD, postoperative cognitive dysfunction; SCWT, Stroop Color Word Test; CLAD-BOS, chronic lung allograft dysfunction-bronchiolitis obliterans syndrome; NHP, Nottingham Health Profile; STAI, State Trait Anxiety Inventory; SDS, Self-rating Depression Scale; IWB, Index of Well-Being; DLT, double lung transplant; SLT, single lung transplant; EMGdi, diaphragm electromyogram; PNCT, phrenic nerve conduction time; CMAPs, compound motor action potentials; ECMO, extracorporeal membrane oxygenation; VV-ECMO, venovenous ECMO; NIV, noninvasive ventilation; ICU, intensive care unit; MV, mechanical ventilation; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; CXR, chest X-ray; IQR, interquartile range; PaCO2, partial pressure of carbon dioxide; HLT, heart-lung transplant; W, Watts; VO2, peak oxygen consumption; HR, heart rate; bpm, beats per minute; ATP, adenosine triphosphate; RLU, relative light units; STRATOS, Strasbourg Thoracic Osteosyntheses System; COVID-19, coronavirus disease 2019; POD, postoperative day; TENS, transcutaneous electrical nerve stimulation; ROM, range of motion.




 PDF
PDF Citation
Citation Print
Print



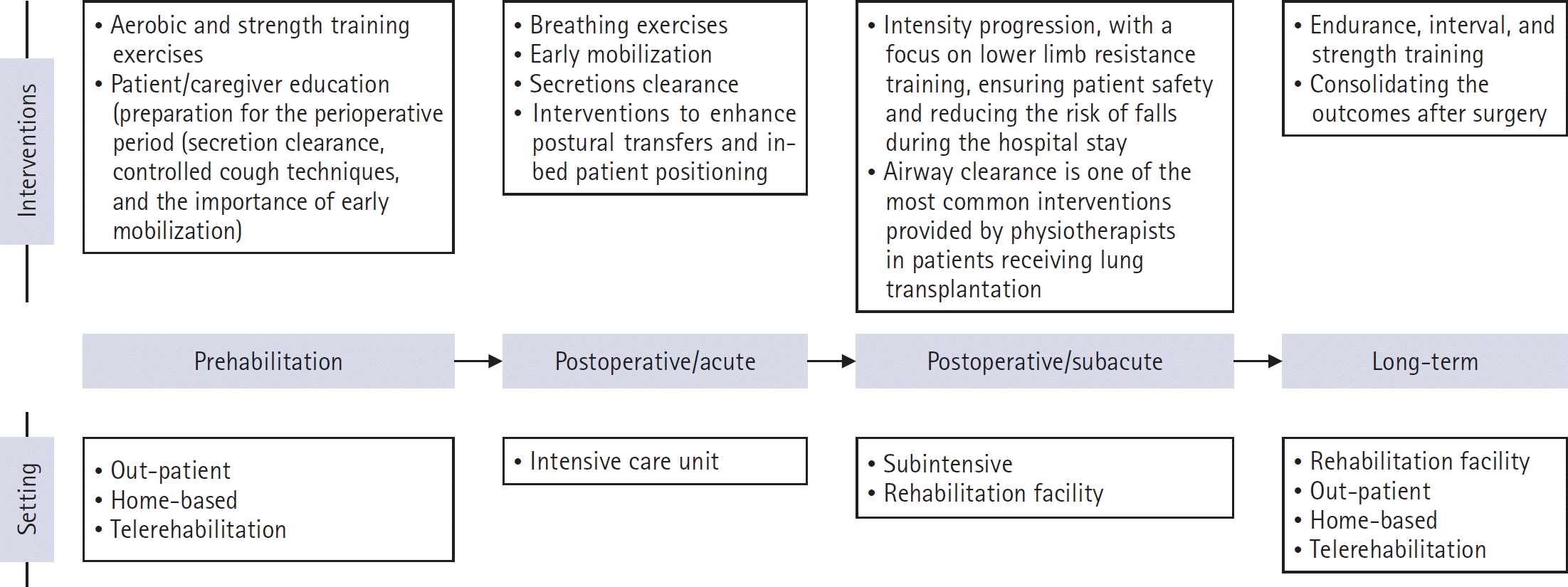
 XML Download
XML Download