Abstract
Cytomegalovirus (CMV) retinitis is a rare disease, and overlapping manifestations involving the anterior segment are extremely uncommon. We report a patient who initially presented with persistent corneal edema and was later diagnosed with CMV retinitis. A 72-year-old man with uncontrolled intraocular pressure (IOP) in his right eye visited a tertiary hospital. At initial presentation, the IOP was 36 mmHg and the fundus was not clear due to corneal edema. Spectral domain optical coherence tomography revealed paracentral acute middle maculopathy (PAMM). Panretinal obstructive vasculopathy was observed on ultra-widefield fluorescein angiography. Three weeks later, trabeculectomy was performed to resolve the persistently high IOP. Once corneal edema improved, a white patch-like peripheral lesion and silver wire-like retinal vasculature were observed. Polymerase chain reaction of the aqueous humor was positive for CMV. Oral valganciclovir and intravitreal ganciclovir were administered as antiviral therapies. Despite treatment for 4 months, the final visual acuity was no light perception, with persistent corneal edema and neovascularization of the iris. We describe a rare case of the simultaneous occurrence of hypertensive uveitis and CMV retinitis. The presence of PAMM could be an initial identifiable sign of CMV retinitis, even in the presence of media opacity.
Cytomegalovirus (CMV) belongs to the Herpesviridae family of double-stranded DNA viruses [1]. It can affect both the anterior and posterior segments of the eye and has a wide range of clinical manifestations [2-4]. CMV-associated anterior uveitis (AU) usually occurs in patients who are immunocompetent [5]. In contrast, typical CMV retinitis is an opportunistic infection found in immunocompromised hosts, such as patients infected with human immunodeficiency virus. Therefore, coexistence of these two diseases in a single individual is uncommon. We report the case of a patient who initially presented with hypertensive uveitis but was later revealed to have CMV retinitis with panretinal occlusive vasculopathy.
Ethics statement: This study was exempted from review by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: 2014-01-019). Written informed consent was obtained from the patient to participate in the study.
A 72-year-old man with uncontrolled intraocular pressure (IOP) in the right eye was referred to our tertiary hospital. He had diabetes mellitus that had been controlled with medication for 20 years. There was no other remarkable medical history. Visual acuity in the right eye was hand motion. The IOP of the right eye was 36 mmHg despite IOP-lowering treatment, including latanoprost eye drops and oral acetazolamide 500 mg three times daily. Slit-lamp examination of the right eye revealed corneal edema with Descemet’s membrane folds. Anterior chamber reactions (cell 1+ and flare 1+) with several fine keratic precipitates were observed. Both lenses showed nuclear sclerosis with anterior and posterior subcapsular cataracts. There were no other remarkable findings in the left eye, and visual acuity was 0.7 (Snellen equivalent). The refractive error was −0.50, and +1.00 diopter respectively. Although the dilated fundus examination was not clear due to corneal haze, it seemed that there were few dot hemorrhages in both eyes (Fig. 1). The cup-to-disc ratio was 0.5 and 0.6 in the right and left eye, respectively. Optical coherence tomography (OCT) revealed hyperreflectivity around the perifovea from the inner to outer plexiform layers in the right eye (Fig. 1). Small cystic spaces and hyperreflective retinal spots suggestive of diabetic retinopathy were observed in the left eye. A general ophthalmologist prescribed 150 mL of 20% mannitol intravenously. One hour after mannitol infusion, the IOP in the right eye dropped to 19 mmHg. The patient was sent home, changing his medication to dorzolamide/timolol fixed combination (twice daily), brimonidine tartrate (twice daily), fluorometholone acetate (0.1%, four times daily), and oral acetazolamide (250 mg, twice daily).
Two days later, the patient underwent a follow-up at the glaucoma clinic. The IOP of the right eye was 31 mmHg, and persistent corneal edema and Descemet’s membrane folds were observed. Other findings were similar to those of the previous visit. Because the OCT findings suggested ischemic damage to the inner nuclear layer (INL), the patient was transferred to the retinal clinic on the same day. A retinal specialist diagnosed paracentral acute middle maculopathy (PAMM). Owing to persistent corneal edema, the fundus was still not clearly visible. The patient was prescribed maximum tolerated medical therapy (MTMT) and was followed up after 1 week.
One week later, the IOP was 24 mmHg in the right eye, and the corneal edema improved slightly. Ultra-widefield fluorescein angiography (FA) was performed. Panretinal obstructive vasculopathy was observed on ultra-widefield FA except in the area surrounding the optic disc (Fig. 2). The retinal specialist prescribed empiric acyclovir 400 mg/day and conducted a blood sample test to evaluate potential infectious causes. Despite MTMT, the IOP was poorly controlled (increased from 24 to 32 mmHg), and trabeculectomy was performed 3 weeks after the first visit.
One week after trabeculectomy, the IOP dropped to 15 mmHg, and the corneal clarity improved. A retinal specialist noticed a white patch lesion with a granular appearance in the temporal area (Fig. 3). Acyclovir was changed to valganciclovir and a diagnostic aqueous tap was performed. Three days later, a polymerase chain reaction (PCR) analysis of the aqueous humor was positive for CMV, and visibility of the fundus through the cornea had improved (Fig. 3). Intravitreal ganciclovir was administered. Three injections of ganciclovir were administered over 2 weeks. After 2 months, the granular retinitis decreased. The retinal specialist recommended pars plana vitrectomy and phacoemulsification to apply panretinal photocoagulation; however, the patient refused for economic reasons. After 15 months, a visual acuity test of the right eye showed no light perception despite 4 months of antiviral therapy. The IOP in the right eye began to increase above 28 mmHg. The patient refused any surgical treatment, except for cataract surgery on the contralateral eye. The right eye showed significant cataracts, diffuse neovascularization on the iris, persistently elevated IOP, and corneal edema at the final visit.
CMV retinitis typically occurs in immunocompromised patients. However, a distinct form of CMV retinitis with panretinal occlusive vasculopathy has been documented under partially immune-dysregulated conditions (e.g., old age and diabetes mellitus) [6-8]. It is characterized by chronic progressive panretinal occlusive vasculopathy and is referred to as chronic retinal necrosis [7]. Although CMV retinitis with panretinal occlusive vasculopathy is rare, it can be identified by a triad of features: granular retinitis, panretinal occlusive vasculopathy, and vitritis [7].
Elevated IOP is a common complication of intraocular inflammation, occurring in 5% to 19% of patients with uveitis [9]. Viral uveitis, including herpes virus infections, often leads to an increased IOP and secondary glaucoma [10,11]. Among viral causes, CMV uveitis is noted to cause the highest IOP levels and represents 9.8% of the cases of hypertensive uveitis [11,12]. Additionally, patients with clinically diagnosed Posner–Schlossman syndrome show IOP stabilization during oral valganciclovir treatment [11]. Therefore, a large proportion of patients with hypertensive uveitis should be considered to have CMV-associated AU.
Theoretically, it is difficult for CMV-associated AU and CMV retinitis to coexist because they occur in different immunologic states. Meanwhile, CMV retinitis with panretinal occlusive vasculopathy appears to be accompanied by mild AU [6,7]. However, no cases of concurrent IOP elevation have been reported previously. To the best of our knowledge, the current study is the first case report of hypertensive uveitis in a patient with CMV retinitis and panretinal occlusive vasculopathy.
Granular appearance is a characteristic finding of typical CMV retinitis and is more frequently observed in the peripheral areas than hemorrhagic appearance [3]. Granular retinitis is also a characteristic feature of CMV retinitis with panretinal occlusive vasculopathy. It was not initially identifiable in the current study because corneal edema reduced the contrast between the normal retina and the retinitis lesion. Thus, alternative findings are needed to diagnose CMV retinitis.
The OCT light source emits longer wavelengths than visible rays, improving penetration through media opacities [13]. OCT can also achieve a high axial resolution that enables the differentiation of individual retinal layers in the human eye in vivo. PAMM is an OCT-based finding described by Sarraf et al. [14]. The hyperreflective lesion around the INL suggests an ischemic event affecting the intermediate and deep capillary plexuses. This finding has been reported in various conditions causing arterial hypoperfusion and is often accompanied by retinal vascular disorders such as central retinal artery occlusion [14,15]. The PAMM served as an important clue in our case as it represented the first abnormal finding detected in the posterior segment. Although not emphasized in previous studies, it appears that all patients undergoing OCT show INL hyperreflectivity as a result of occlusive vasculopathy (Table 1) [6-8].
In our case, the patient exhibited several complications at the last visit, including neovascularization of the iris, persistent IOP elevation, and corneal edema. Patients with CMV AU commonly present with advanced glaucoma or irreversible corneal edema, which may require corneal transplantation [16]. Large nonperfusion areas may be associated with the development of neovascularization in patients with CMV retinitis and panretinal occlusive vasculitis [6,17,18]. Schneider et al. [7] reported that neovascularization occurred in four out of five patients in their case series. Long et al. [17] noted that some cases of neovascularization can progress to neovascular glaucoma (NVG). Ischemia in the retina leads to the release of angiogenic factors, resulting in neovascularization of the iris and the subsequent development of NVG [19]. This neovascularization can obstruct the trabecular meshwork, impeding aqueous humor outflow, and causing a significant and sustained increase in IOP. Prolonged IOP elevation can disrupt the barrier function of the corneal endothelium, leading to decompensation, which can eventually progress to irreversible corneal edema [20].
Due to its rare clinical prevalence, delayed (from 7 to 50 days) or inappropriate treatments are common in CMV retinitis with panretinal occlusive vasculopathy [7]. Early antiviral therapy is known to prevent the progression of granular retinitis [6]. However, occlusive vasculopathy has been reported to progress despite antiviral treatment, leading to poor visual prognosis [6,7]. Local or systemic steroids after initiation of antiviral therapy seemed to improve occlusive vasculopathy in previous studies [6,7]. Therefore, early detection is important in the management of CMV retinitis with panretinal occlusive vasculopathy.
The triad of CMV retinitis with panretinal occlusive vasculitis includes vitritis, granular retinitis, and occlusive vasculitis [7]. In our case, the elevated IOP led to corneal edema, making it difficult to recognize the granular retinitis, which is usually observed in the peripheral retina. However, vitritis was detectable, and occlusive vasculitis was evident on OCT imaging. Previous studies have shown that CMV is causative in approximately 10% of eyes with hypertensive uveitis [11]. Therefore, when hypertensive uveitis is accompanied by vitritis and PAMM, diagnostic PCR should be considered to confirm the CMV infection.
In summary, the presence of PAMM in eyes with hypertensive uveitis could be a supportive indication for CMV retinitis in immunocompetent patients, which is more valuable when corneal edema interferes with posterior segment visualization during fundus examination. When PAMM is identified by OCT in patients with hypertensive uveitis, empiric antiviral treatments with or without steroids should be considered to prevent the deterioration of visual acuity.
References
1. Hirai K. The mechanisms of human cytomegalovirus DNA replication. Nihon Rinsho. 1998; 56:36–43.
2. Port AD, Orlin A, Kiss S, Patel S, D'Amico DJ, Gupta MP. Cytomegalovirus retinitis: a review. J Ocul Pharmacol Ther. 2017; 33:224–34.
3. Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for cytomegalovirus retinitis. Am J Ophthalmol. 2021; 228:245–54.
4. Kempen JH, Min YI, Freeman WR, Holland GN, Friedberg DN, Dieterich DT, et al. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006; 113:684–94.
5. Ye Z, Yang Y, Ke W, Li Y, Wang K, Chen M. Overview and update on cytomegalovirus-associated anterior uveitis and glaucoma. Front Public Health. 2023; 11:1117412.
6. Wang I, Yang BC, Li KH, Wu JS, Chen SN. CMV retinitis with panretinal occlusive vasculitis. Ocul Immunol Inflamm. 2024; 32:477–84.
7. Schneider EW, Elner SG, van Kuijk FJ, Goldberg N, Lieberman RM, Eliott D, et al. Chronic retinal necrosis: cytomegalovirus necrotizing retinitis associated with panretinal vasculopathy in non-HIV patients. Retina. 2013; 33:1791–9.
8. Izzo MC, Mathai M, Do BK. Cytomegalovirus retinitis without immunocompromise. Retina Today. 2021; May/June. 53–5.
9. Moorthy RS, Mermoud A, Baerveldt G, Minckler DS, Lee PP, Rao NA. Glaucoma associated with uveitis. Surv Ophthalmol. 1997; 41:361–94.
10. Lewkowicz D, Willermain F, Relvas LJ, Makhoul D, Janssens S, Janssens X, et al. Clinical outcome of hypertensive uveitis. J Ophthalmol. 2015; 2015:974870.
11. Pohlmann D, Pahlitzsch M, Schlickeiser S, Metzner S, Lenglinger M, Bertelmann E, et al. Virus-associated anterior uveitis and secondary glaucoma: diagnostics, clinical characteristics, and surgical options. PLoS One. 2020; 15:e0229260.
12. Sobolewska B, Deuter C, Doycheva D, Zierhut M. Long-term oral therapy with valganciclovir in patients with Posner-Schlossman syndrome. Graefes Arch Clin Exp Ophthalmol. 2014; 252:117–24.
13. Aumann S, Donner S, Fischer J, Müller F. Optical coherence tomography (OCT): principle and technical realization. In : Bille JF, editor. High resolution imaging in microscopy and ophthalmology: new frontiers in biomedical optics. Cham, Swiss: Springer International Publishing;2019. p. 59–85.
14. Sarraf D, Rahimy E, Fawzi AA, Sohn E, Barbazetto I, Zacks DN, et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013; 131:1275–87.
15. Liang S, Chen Q, Hu C, Chen M. Association of paracentral acute middle maculopathy with visual prognosis in retinal artery occlusion: a retrospective cohort study. J Ophthalmol. 2022; 2022:9404973.
16. Kam KW, Wong CH, Ho M, Sze RK, Chan PK, Young AL. Iris depigmentation in the prediction of cytomegalovirus anterior uveitis. Ocul Immunol Inflamm. 2022; 30:1775–80.
17. Long Z, Hou J, Miao H. Neovascular complications from cytomegalovirus necrotizing retinopathy in patients after haploidentical hematopoietic stem cell transplantation. Retina. 2021; 41:1526–32.
18. Park SH, Cho H, Seong M, Kang MH, Shin YU. Rapidly progressive neovascular glaucoma associated with atypical cytomegalovirus retinitis: a case report. Korean J Ophthalmol. 2022; 36:171–9.
19. Tang Y, Shi Y, Fan Z. The mechanism and therapeutic strategies for neovascular glaucoma secondary to diabetic retinopathy. Front Endocrinol (Lausanne). 2023; 14:1102361.
20. Guzun OV, Drozhzhyna GI. Comprehensive treatment of patients with refractory glaucoma complicated by bullous keratopathy. J Ophthalmol (Ukraine). 2019; 1:17–22.
Fig. 1.
(A) Fundus photography and (B) optical coherence tomography (OCT) at initial presentation. Corneal edema hinders imaging in the right eye. OCT shows a hyperreflective band-like lesion (arrows) in the inner plexiform layer, inner plexiform layer, and outer plexiform layer.
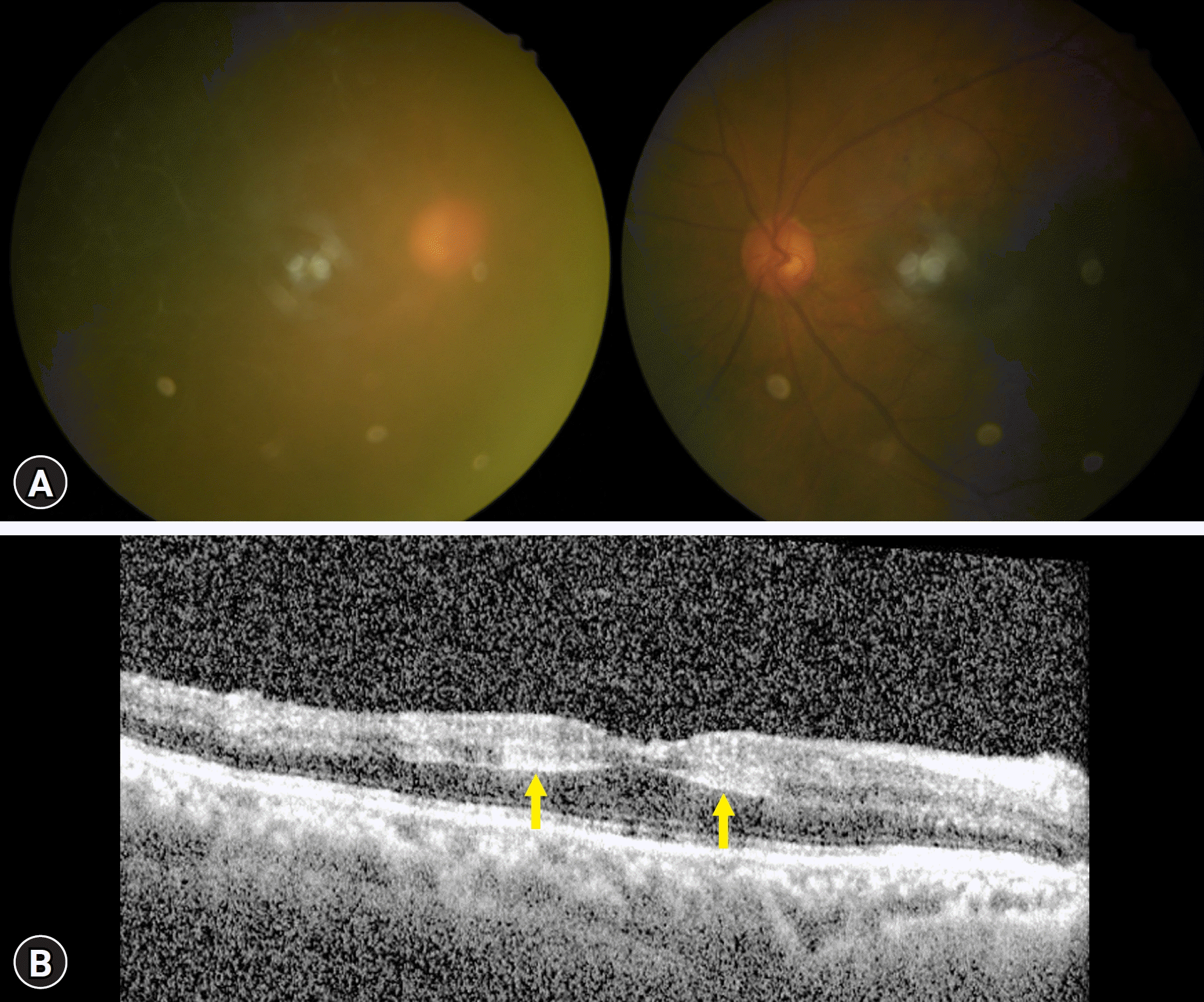
Fig. 2.
Ultra-widefield fluorescein angiography after 1 week. The right eye shows panretinal vascular occlusion, although it is not clearly visible due to persistent corneal edema.
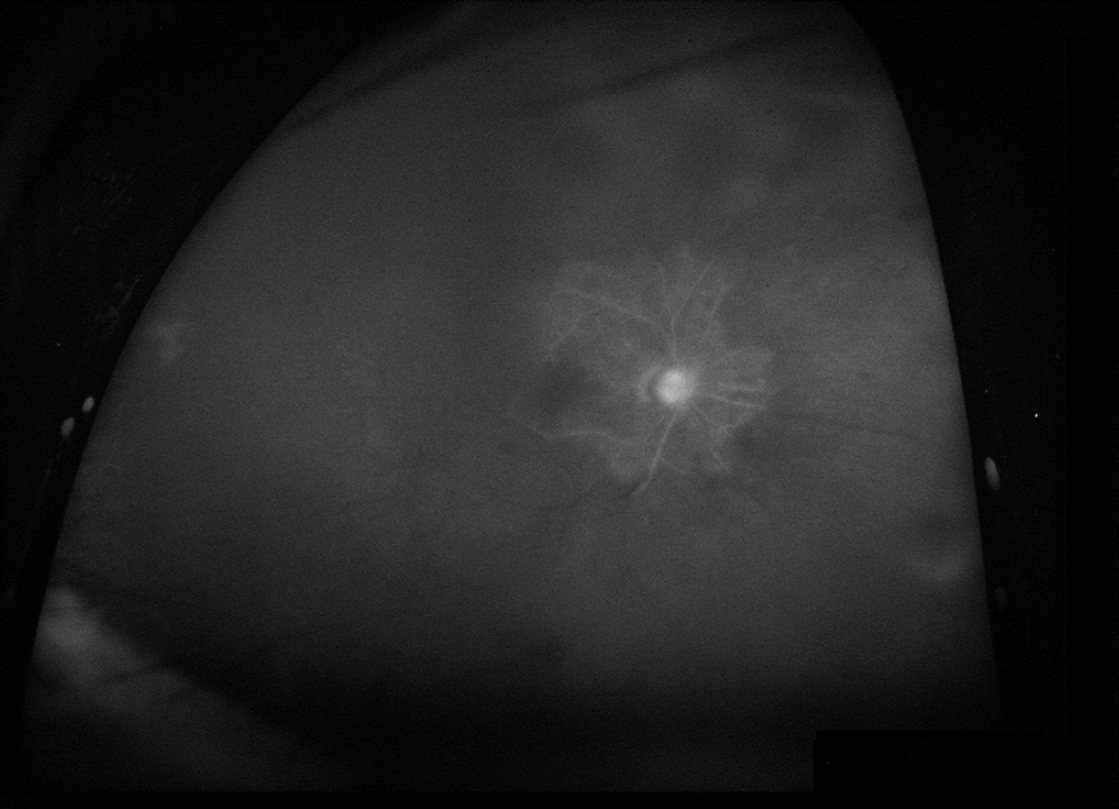
Fig. 3.
Ultra-widefield image after trabeculectomy. (A) White lesion with granular appearance is observed through improved corneal edema. (B) Three days later, the fundus is more clearly visible, showing the characteristic granular appearance of cytomegalovirus retinitis.
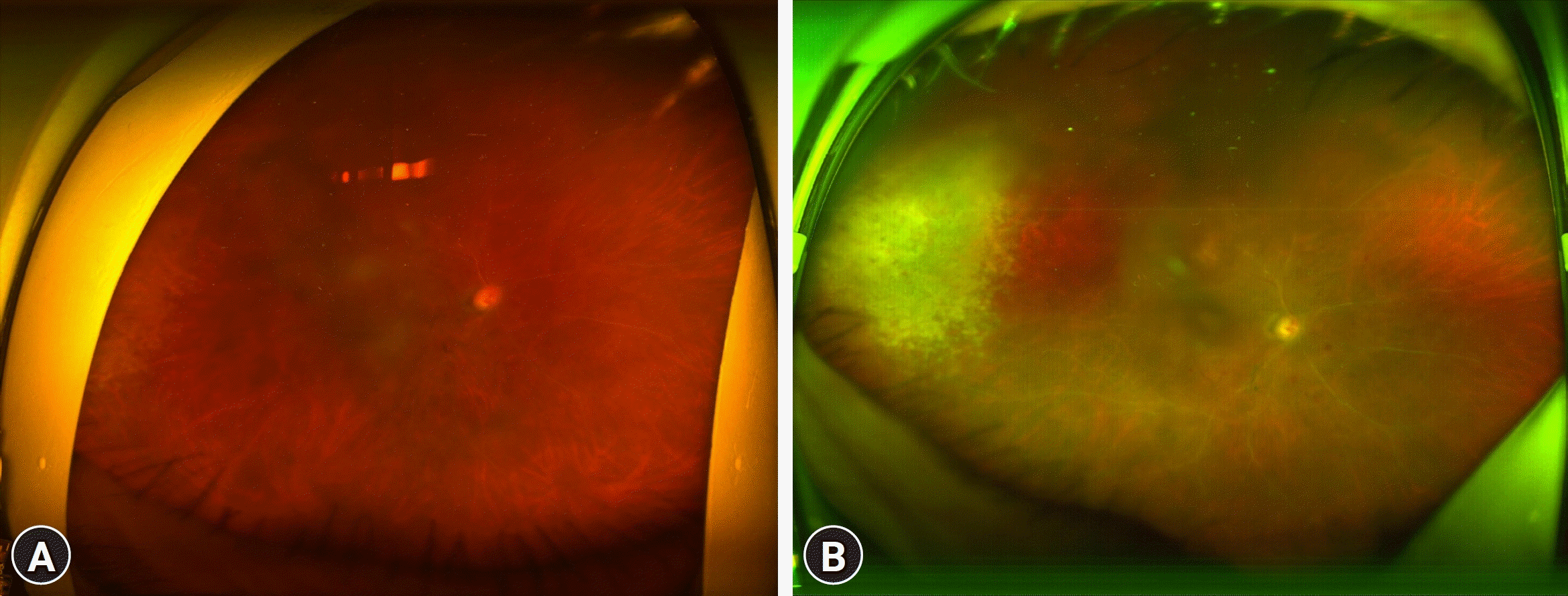
Table 1.
Summary of previous case reports describing cytomegalovirus retinitis with panretinal occlusive vasculopathy
| Study | Case No. | Age (yr)/sex | Underlying disease | Immunosuppressant | Initial VA | Final VA | AC cell | Vitritis/POV/GR | PAMM |
|---|---|---|---|---|---|---|---|---|---|
| Schneider et al. [7] | 1 | 74/Male | DM, CKD, post-KT 9 years | MMF (750 mg twice daily) | 20/30 | HM | 1+ | +/+/+ | NAa) |
| Tacrolimus 1 mg twice daily | |||||||||
| 2 | 83/Female | DM, HTN, CAD, anemia | - | HM | HM | 1+ | +/+/+ | NAa) | |
| 3 | 48/Male | MM (post-BM transplant) | Corticosteroid tacrolimus | 20/25 | 20/20 | 2–3+ | +/+/+ | NAa) | |
| GVH, steroid-induced DM | |||||||||
| 4 | 78/Male | DM | - | 20/30 | 20/30 | Trace | +/+/+ | NAa) | |
| 5 | 72/Male | Polymyositis | Prednisolone | 5/200 | 20/40 | 2+ | +/+/+ | NAa) | |
| Wang et al. [6] | 1 | 71/Male | DM | - | 0.1 | 0.02 | 1+ | +/+/+ | + |
| 2 | 66/Male | DM (poorly controlled) | Subtenon triamcinolone per 2–3 months | 0.02 | HM | Trace | +/+/+ | NAa) | |
| 3 | 58/Male | MM, hematopoietic cell transplant | Dexamethasone | 0.1 | 0.3 | 1+ | +/+/+ | + | |
| Izzo et al. [8] | 1 | 71/Male | Prostate cancer (no RT or chemotherapy) | - | FC | Not described | 1+ | +/+/+ | + |
| Sarcoidosis (no treatment) |
VA, visual acuity; AC, anterior chamber; POV, panretinal obstructive vasculopathy; GR, granular retinitis; PAMM, paracentral acute middle maculopathy; DM, diabetes mellitus; CKD, chronic kidney disease; KT, kidney transplantation; MMF, mycophenolate mofetil; HM, hand movement; NA, not applicable; HTN, hypertension; CAD, coronary artery disease; MM, multiple myeloma; BM, bone marrow; GVH, graft versus host disease; RT, radiation therapy; FC, finger count.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download