Abstract
Background
Methods
Results
Supplementary Material
Supplemental Table S1.
Supplemental Table S2.
Supplemental Table S3.
Supplemental Table S4.
Notes
CONFLICTS OF INTEREST
Chang Ho Ahn in Lunit as a medical director and has a stock option in the firm. Jung Hee Kim is a deputy editor of the journal. But she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
Conception or design: S.H.L., J.H.K. Acquisition, analysis, or interpretation of data: S.K.B., S.H.L., S.S.P., C.H.A., S.H.K., W.W.K., Y.M.L., S.J.K., D.E.S., T.Y.S., K.E.L., J.H.K., K.C.J., J.M.K. Drafting the work or revising: S.K.B., S.H.L., J.H.K. Final approval of the manuscript: S.K.B., S.H.L., S.S.P., C.H.A., S.H.K., W.W.K., Y.M.L., S.J.K., D.E.S., T.Y.S., K.E.L., J.H.K., K.C.J., J.M.K.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
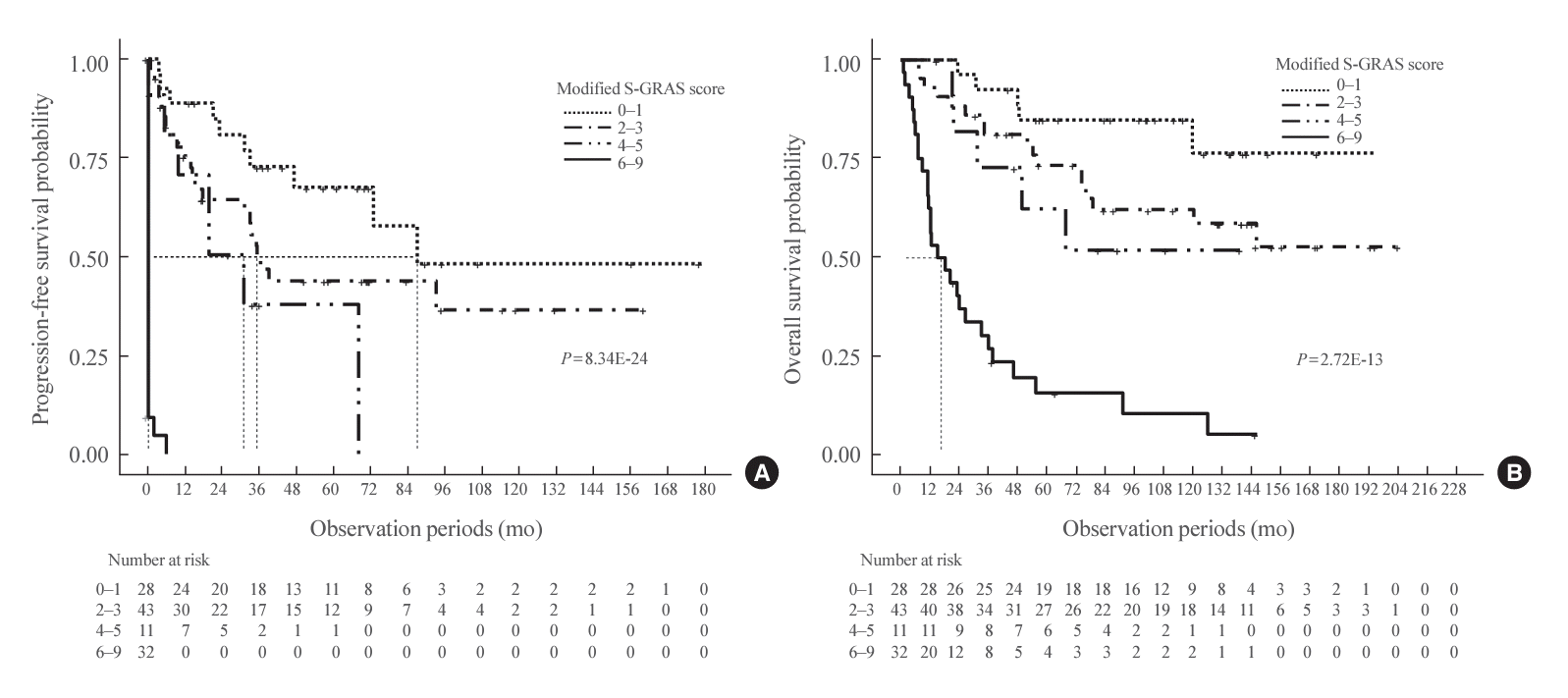
Table 1.
| Variable | Available | Value |
|---|---|---|
| Women | 114 (100.0) | 67 (58.8) |
| Age at diagnosis, yr | 114 (100.0) | 51.0 (41.0–62.0) |
| ≥50 | 114 (100.0) | 61 (53.5) |
| BMI, kg/m2 | 105 (92.1) | 23.6 (22.0–25.9) |
| Size, cm | 111 (97.4) | 7.9 (5.5–11.0) |
| Symptom | 111 (97.4) | 55 (48.2) |
| ENSAT stage | 114 (100.0) | |
| 1 | 13 (11.4) | |
| 2 | 54 (47.4) | |
| 3 | 17 (14.9) | |
| 4 | 30 (26.3) | |
| Ki67 index or mitotic count | ||
| Ki67 index | 79 (69.3) | 10.0 (4.0–18.5) |
| Mitotic counts/50 HPF | 96 (84.2) | 20.0 (5.0–46.5) |
| Additional treatmentb | 114 (100.0) | 61 (53.5) |
| Adjuvant mitotane | 114 (100.0) | 35 (30.7) |
| Mitotane dose, mg | 32 (94.1) | 2,000 (1,500–3,000) |
| Mitotane level, mg/L | 9 (26.5) | 11.8 (7.1–13.5) |
| Mitotane blood level >14 mg/L | 9 (26.5) | 6 (66.7) |
| Resection status | 114 (100.0) | |
| R0 | 70 (61.4) | |
| RX | 7 (6.1) | |
| R1 | 7 (6.1) | |
| R2 | 30 (26.3) |
Values are expressed as number (%) or median (interquartile range).
ACC, adrenocortical carcinoma; mS-GRAS, modified Stage, Grade, Resection status, Age, Symptom; BMI, body mass index; ENSAT, European Network for the Study of Adrenal Tumour; HPF, high powered field.
Table 2.
| Scores | Variable | No. (%) | No. of relapse (%) | HR (95% CI) | P value | Harrell’s C-index (95% CI) | P valuec | P valued |
|---|---|---|---|---|---|---|---|---|
| S | ENSAT stage | 114 (100.0) | 70 (61.4) | 0.782 (0.716‒0.833)e | 0.037e | 0.115 | ||
| 0 | 1‒2 | 67 (58.8) | 33 (49.3) | Ref | ||||
| 1 | 3 | 17 (14.9) | 7 (41.2) | 1.12 (0.49–2.56) | 0.786 | |||
| 2 | 4 | 30 (26.3) | 30 (100.0) | NA | 0.994 | |||
| Modified G | Ki67 index (%) or mitotic count, mitoses/50 HPF | 114 (100.0) | 70 (61.4) | 0.644 (0.558‒0.715)e | <0.001e | <0.001e | ||
| 0 | 0‒9 or ≤5 | 44 (38.6) | 21 (47.7) | Ref | ||||
| 1 | 10‒19 or 6‒20 | 33 (28.9) | 21 (63.6) | 1.67 (0.91–3.07) | 0.097 | |||
| 2 | ≥20 or >20 | 37 (32.5) | 28 (75.7) | 2.99 (1.67–5.35)e | <0.001e | |||
| R | Resection status | 114 (100.0) | 70 (61.4) | 0.814 (0.755‒0.867)e | 0.333 | 0.832 | ||
| 0 | R0 | 70 (61.4) | 30 (42.9) | Ref | ||||
| 1 | RX | 7 (6.1) | 6 (85.7) | 3.29 (1.35–8.03)e | 0.009e | |||
| 2 | R1 | 7 (6.1) | 4 (57.1) | 2.15 (0.76–6.14) | 0.151 | |||
| 3 | R2 | 30 (26.4) | 30 (100.0) | NA | 0.995 | |||
| A | Age, yr | 114 (100.0) | 70 (61.4) | 0.526 (0.480‒0.583) | <0.001e | <0.001e | ||
| 0 | <50 | 54 (47.4) | 35 (64.8) | Ref | ||||
| 1 | ≥50 | 60 (52.6) | 35 (58.3) | 0.89 (055–1.42) | 0.614 | |||
| S | Symptom | 114 (100.0) | 70 (61.4) | 0.628 (0.563‒0.691)e | <0.001e | <0.001e | ||
| 0 | No | 60 (52.6) | 28 (46.7) | Ref | ||||
| 1 | Yes | 54 (47.4) | 42 (77.8) | 2.26 (1.40–3.65)e | 0.001e | |||
| mS-GRAS | 114 (100.0) | 70 (61.4) | 1.90 (1.65–2.18)e | <0.001e | 0.829 (0.759‒0.880)e | Ref | ||
| mS-GRAS group | 114 (100.0) | 70 (61.4) | 0.815 (0.751‒0.864)e | Ref | ||||
| 0–1 | 28 (24.6) | 10 (35.7) | Ref | |||||
| 2–3 | 43 (37.7) | 22 (51.2) | 1.84 (0.87–3.90) | 0.109 | ||||
| 4–5 | 11 (9.6) | 7 (63.6) | 3.18 (1.19–8.48)e | 0.021e | ||||
| 6–9 | 32 (28.1) | 31 (96.9) | 69.51 (25.99–185.86)e | <0.001e |
PFS, progression-free survival; HR, hazard ratio; mS-GRAS, modified Stage, Grade, Resection status, Age, Symptom; CI, confidence interval; ENSAT, European Network for the Study of Adrenal Tumour; HPF, high powered field; R0, complete resection; RX, uncertain resection; R1, microscopic incomplete resection; R2, macroscopic incomplete resection.
a mS-GRAS components: ENSAT stage (S), modified grading (modified G), resection status (R), age (A), and tumor- or hormone-related symptoms (S);
Table 3.
| Scores | Variable | No. (%) | No. of death (%) | HR (95% CI) | P value | Harrell’s C-index (95% CI) | P valuec | P valued |
|---|---|---|---|---|---|---|---|---|
| S | ENSAT stage | 114 (100.0) | 55 (48.2) | 0.700 (0.627‒0.754)e | 0.047e | 0.050e | ||
| 0 | 1‒2 | 67 (58.2) | 22 (32.8) | Ref | ||||
| 1 | 3 | 17 (14.9) | 8 (47.1) | 2.36 (0.62–8.94) | 0.205 | |||
| 2 | 4 | 30 (26.3) | 25 (83.3) | 8.23 (2.47–27.50)e | 0.001e | |||
| Modified G | Ki67 index (%) or mitotic count, mitoses/50 HPF | 114 (100.0) | 55 (48.2) | 0.634 (0.553‒0.694)e | 0.001e | 0.004e | ||
| 0 | 0‒9 or ≤5 | 44 (38.6) | 14 (31.8) | Ref | ||||
| 1 | 10‒19 or 6‒20 | 33 (28.9) | 14 (42.4) | 1.41 (0.67–2.96) | 0.365 | |||
| 2 | ≥20 or >20 | 37 (32.5) | 27 (73.0) | 3.44 (1.79–6.58)e | <0.001e | |||
| R | Resection status | 114 (100.0) | 55 (48.2) | 0.713 (0.636‒0.769)e | 0.148 | 0.141 | ||
| 0 | R0 | 70 (61.4) | 21 (30.0) | Ref | ||||
| 1 | RX | 7 (6.1) | 5 (71.4) | 2.91 (1.09–7.75)e | 0.033e | |||
| 2 | R1 | 7 (6.1) | 4 (57.1) | 2.39 (0.82–6.96) | 0.112 | |||
| 3 | R2 | 30 (26.4) | 25 (83.3) | 6.33 (3.48–11.50)e | <0.001e | |||
| A | Age, yr | 114 (100.0) | 55 (48.2) | 0.545 (0.490‒0.612) | <0.001e | <0.001e | ||
| 0 | <50 | 54 (47.4) | 22 (40.7) | Ref | ||||
| 1 | ≥50 | 60 (52.6) | 33 (55.0) | 1.56 (0.91–2.69) | 0.106 | |||
| S | Symptom | 114 (100.0) | 55 (48.2) | 0.551 (0.484‒0.613) | <0.001e | <0.001e | ||
| 0 | No | 60 (52.6) | 27 (45.0) | Ref | ||||
| 1 | Yes | 54 (47.4) | 28 (51.9) | 1.32 (0.78–2.25) | 0.298 | |||
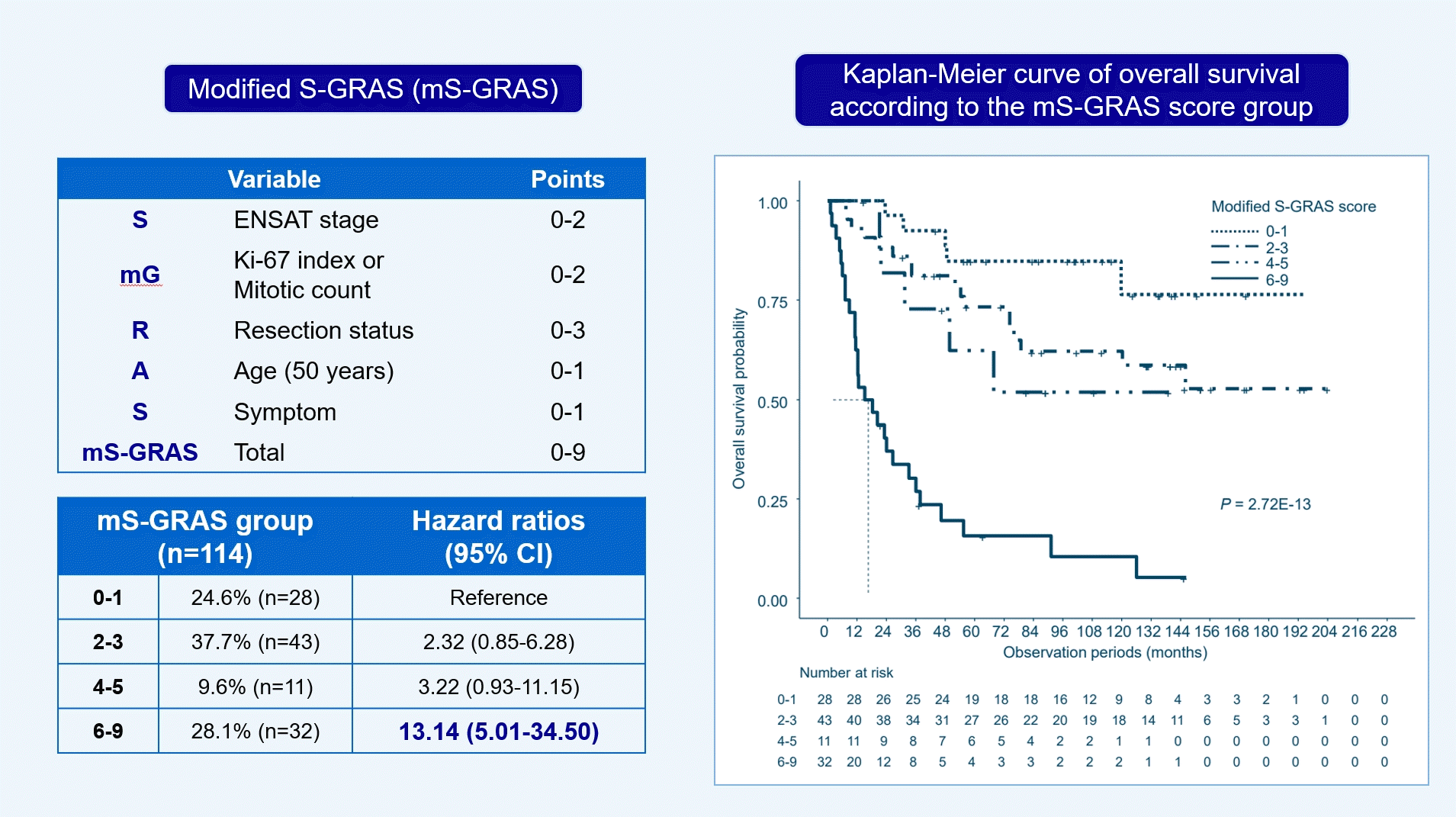
| mS-GRAS | 114 (100.0) | 55 (48.2) | 1.37 (1.25–1.50)e | <0.001e | 0.747 (0.684‒0.805)e | Ref | ||
| mS-GRAS group | 114 (100.0) | 55 (48.2) | 0.746 (0.673‒0.795)e | Ref | ||||
| 0–1 | 28 (24.6) | 5 (17.9) | Ref | |||||
| 2–3 | 43 (37.7) | 17 (39.5) | 2.32 (0.85–6.28) | 0.099 | ||||
| 4–5 | 11 (9.6) | 5 (45.5) | 3.22 (0.93–11.15) | 0.065 | ||||
| 6–9 | 32 (28.1) | 28 (87.5) | 13.14 (5.01–34.50)e | <0.001e |
OS, overall survival; HR, hazard ratio; mS-GRAS, modified Stage, Grade, Resection status, Age, Symptom; CI, confidence interval; ENSAT, European Network for the Study of Adrenal Tumour; HPF, high powered field; R0, complete resection; RX, uncertain resection; R1, microscopic incomplete resection; R2, macroscopic incomplete resection.
a mS-GRAS components: ENSAT stage (S), modified grading (modified G), resection status (R), age (A), and tumor- or hormone-related symptoms (S);




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download