Abstract
Background
Methods
Conclusion
Notes
AUTHOR CONTRIBUTIONS
Conception or design: K.C.W., Y.S.C., S.G.K. Revision of study protocol: N.H.K., J.L., S.C., J.M.Y., I.K.J., S.L., W.J.K., K.S., H.C.C., H.M.Y., K.A.K., S.S.K., S.H.L., C.H.K., S.H.K., Y.L., C.H.C., S.L., H.Y.J., J.H.L., G.K., S.Y.K., J.K., J.H.L., T.N.K., H.J.J., J.H.L., J.H.J., H.J.Y., H.K.K., H.K.P., I.S.N.G., S.H., C.W.A., J.H.Y., J.H.P., K.G.P., C.H.P., K.H.J., O.H.R., K.Y.P., E.G.H., B.S.C., K.C.W., Y.S.C., S.G.K. Drafting the work or revising: N.H.K., J.L., K.C.W., Y.S.C., S.G.K. Final approval of the manuscript: K.C.W., Y.S.C., S.G.K.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
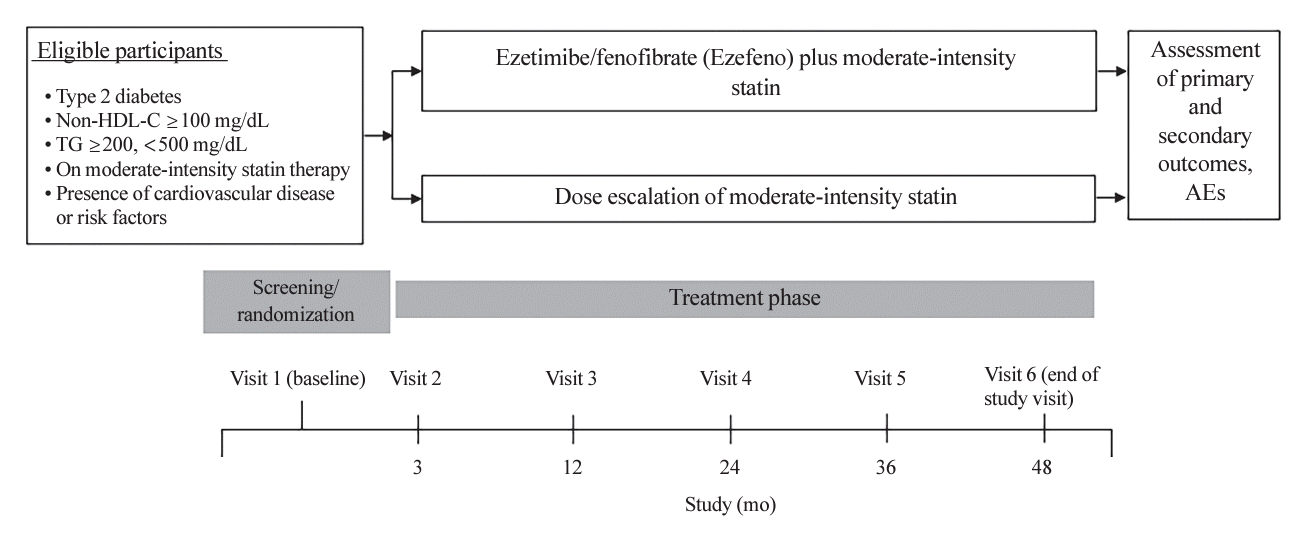
Table 1.
| Inclusion criteria |
| Patients diagnosed with type 2 diabetes according to American Diabetes Association criteria |
| Age ≥19 years |
| Non-HDL-C ≥100 mg/dL, TG ≥200, <500 mg/dL on moderate-intensity statins |
| Presence of cardiovascular disease or at least one of cardiovascular risk factorsa |
| Exclusion criteria |
| Pregnant or breastfeeding women |
| Uncontrolled hyperglycemia (HbA1c >12.0%) |
| Serum creatinine >2.5 mg/dL or eGFR <30 mL/min/1.73 m2 |
| Gall bladder disease, myopathy, or rhabdomyolysis requiring treatment |
| Elevated liver enzymes (AST, ALT) >3 times the upper normal limit |
| Hypersensitivity to study drugs |
HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HbA1c, glycosylated hemoglobin; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
a Cardiovascular diseases include coronary artery disease, ischemic stroke, transient ischemic attack, carotid artery disease, and peripheral artery disease. Cardiovascular risk factor includes abdominal aortic aneurysm, duration of diabetes ≥10 years, age (men ≥45 years, women ≥55 years), family history of premature atherosclerotic cardiovascular disease, hypertension, smoking, low HDL-C level (<40 mg/dL), albuminuria, chronic kidney disease (eGFR <60 mL/min/1.73 m2), retinopathy, neuropathy, left ventricular hypertrophy.
Table 2.
| Variable | Screening/baselinea (visit 1) | Randomization | Visit 2 (3 mo) | Visit 3 (12 mo) | Visit 4 (24 mo) | Visit 5 (36 mo) | Visit 6 (48 mo) |
|---|---|---|---|---|---|---|---|
| Visit windows, day | ±1 mo | ±3 mo | ±3 mo | ±3 mo | ±3 mo | ||
| Written informed consent | X | ||||||
| Demographicsb | X | ||||||
| Physical examination | X | ||||||
| Vital sign | X | X | X | X | X | X | |
| Past medical history | X | X | X | X | X | X | |
| 12-lead ECG | X | X | X | X | X | X | |
| Inclusion/exclusion criteria | X | ||||||
| Randomization to treatment groups | X | ||||||
| Laboratory testc | X | X | X | X | X | X | |
| Assessment of trial outcomes/adverse events | X | X | X | X | X | X |
b Demographic information includes age, date of birth, sex, height, weight, waist circumference, smoking history, alcohol consumption, and physical activity level;
c Laboratory tests including hematology (red blood cell count, white blood cell [WBC] count, hemoglobin, hematocrit, platelet, WBC differential count, high-sensitivity C-reactive protein), blood chemistry tests (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma glutamyl transpeptidase, creatinine phosphokinase, total bilirubin, creatinine), urinalysis (pH, specific gravity, protein, WBC), urine albumin/creatinine, blood glucose test (glycosylated hemoglobin, fasting plasma glucose, fasting insulin), lipid profile (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol [HDL-C], triglyceride, non-HDL-C) will be measured at the site visits or recorded from electronic medical records.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download