Abstract
Doxylamine is an over-the-counter first-generation antihistamine used in the treatment of allergies and insomnia. Intentional ingestion is common due to its wide availability. Toxicity most frequently presents as an anticholinergic toxidrome with central effects leading to delirium, psychosis, or seizures, and peripheral effects manifested as tachycardia, dry and warm skin, mydriasis, or urinary retention. First-generation antihistamines can have antagonistic properties against the sodium channels on the cardiac myocytes in supratherapeutic doses. This article reports a polysubstance ingestion of 2 antihistaminergic agents with development of QRS complex widening and a right bundle branch block, which has not been well described in the literature.
Doxylamine, N,N-Dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine, is a first-generation, over-the-counter antihistamine used in the treatment of respiratory allergies and as a sleep aid (1). In cases of overdose (or poisoning), it can lead to severe or rarely fatal toxicity due to its anticholinergic effects. Doxylamine overdose usually manifests as a classic antimuscarinic toxidrome with mydriasis, agitation, hallucinations, dry membranes, or urinary retention (1-3). Other reported complications include rhabdomyolysis, pancreatitis, acute kidney injury, and syndrome of inappropriate antidiuretic hormone (4-6).
First-generation antihistamines, such as diphenhydramine, doxylamine, or pyrilamine, are known to have sodium channel-blocking effects in massive overdose. Pediatricians or emergency physicians use the prolongation of QRS complex as a marker for the effects. This is similar to those seen in class I antiarrhythmics (e.g., quinidine) or anesthetics (e.g., lidocaine) (7). However, to the authors’ best knowledge, there has been a lack of literature reporting the doxylamine-induced sodium channel toxicity. This case report describes the widening of QRS complex in the setting of a doxylamine and pyrilamine maleate overdose.
A 17-year-old female with a recent diagnosis of major depressive disorder presented to an outside hospital emergency department (ED) after an intentional overdose. She was reportedly well until found in a bathroom with generalized tonic-clonic movements and a transient alteration in awareness for which an emergency medical service team was immediately called with concern for seizure activity. Three empty pill bottles were found beside her: UnisomTM (Sanofi Consumer Healthcare North America; 48 tablets of doxylamine, 25 mg), Menstrual CompleteTM (Healthlife of USA; 40 tablets of acetaminophen 500 mg, pyrilamine maleate 15 mg, and caffeine 60 mg), and fluoxetine (28 tablets of 20 mg). The estimated total amount of ingested medications was 1.2 g of doxylamine (17.5 mg/kg), 20 g of acetaminophen (292 mg/kg), 600 mg of pyrilamine maleate (8.7 mg/kg), 2.4 g of caffeine (35 mg/kg), and 560 mg of fluoxetine (8 mg/kg). On arrival at the ED, the patient was agitated, altered, and tachycardic. Initial vital signs were as follows: blood pressure, 125/85 mmHg; heart rate, 146 beats/minute; respiratory rate, 16 breaths/minute; temperature, 36.8 °C; oxygen saturation, 94% on room air; and a Glasgow Coma Scale of 14 (eye opening: 4, verbal response: 4, and motor response: 6). The initial electrocardiogram (ECG) demonstrated QRS complex widening at 23:30 on day 1 (Fig. 1). Initial laboratory workup is shown in Table 1. No neuroimaging was performed at the ED.
Initial laboratory findings showed a mildly elevated concentrations of creatine kinase (CK), creatinine, and acetaminophen measured after approximately 5 hours of ingestion (Table 1). She received intravenously 2 L of 0.9% saline bolus and started on N-acetylcysteine (NAC) infusion as per the 21-hour intravenous protocol. A local poison center recommended 2 ampules of sodium bicarbonate (1 ampule is 50 mL of 1 mEq/mL) against the QRS widening and lorazepam against agitation. After the administration of sodium bicarbonate, a repeat ECG showed improvement in the QRS interval and an incomplete right bundle branch block at 00:39 on day 2 of hospitalization (Fig. 2). Two more ampules of sodium bicarbonate were given, and she was started on a sodium bicarbonate continuous infusion (150 mEq in 1 L 5% dextrose in water at a rate of 150 mL/hour). The patient was then transferred to our pediatric intensive care unit (PICU).
Upon arrival to the PICU at 02:50 on day 2, the patient remained at a heart rate of 120-140 beats/minute after the prior 2 L of intravenous 0.9% saline, and was noted to be incontinent of urine. Her physical examination showed active hallucinations with the patient grabbing at air, bilateral horizontal nystagmus with mydriasis to 8 mm pupil size. She had dry mucous membranes and 2-3 beats of clonus in the lower extremities without hyperreflexia. A repeat ECG showed resolution of the abovementioned abnormalities at 04:28 on day 2 (Fig. 3); subsequently, the sodium bicarbonate infusion was discontinued. The NAC infusion was also discontinued, as she had 2 undetectable acetaminophen concentrations without liver function derangements and thus met early stop criteria for NAC infusion.
The patient continued to hallucinate until approximately 36 hours after ingestion. Given an increase in CK to 11,568 U/L, she was started on 0.9% saline at 160 mL/hour for treatment of rhabdomyolysis, thereby leading to improvement of CK and creatinine. Approximately 36 hours after ingestion, she began following commands and was no longer requiring lorazepam for her safety. She was transferred out of the PICU on day 3 and evaluated by psychiatry for hospitalization. She did not develop signs or symptoms of acetaminophen-induced hepatotoxicity or serotonin syndrome.
The case patient ingested 17.5 mg/kg of doxylamine as well as 8.7 mg/kg of pyrilamine maleate, another first-generation antihistamine, along with multiple other substances. The dose was lower than 20 mg/kg, which can predict the development of doxylamine-induced rhabdomyolysis with an 81% sensitivity and 82% specificity (4). The smaller dose required to cause the electrocardiographic abnormalities may be due to her co-ingestion. The patient exhibited classic toxidromes of doxylamine overdose, such as tachycardia, psychosis, hallucinations, dry skin, rhabdomyolysis, and mydriasis, but had no further movements concerning seizures.
Her tachycardia was likely multifactorial. Any antimuscarinic toxidrome can cause tachycardia. Fluoxetine can contribute to serotonin toxicity, which includes tachycardia. In addition, caffeine can also induce tachycardia via adenosine receptor blockade and subsequent increase in concentration of catecholamines.
As this was a multidrug overdose, the QRS complex widening cannot be definitively attributed to doxylamine alone and is potentially related to co-ingestants such as pyrilamine maleate (8). Her vital signs and physical exam were most consistent with antimuscarinic ingestion while the other agents described, including fluoxetine, caffeine, and acetaminophen, lack sodium channel blocking potential. It is theorized that large quantities of antimuscarinic agents can interact with sodium channels leading to their blockade. Given that diphenhydramine is well described to have this effect (9), it is reasonable to assume that an agent with a similar structure and in the same pharmacologic class would have this potential as well. In addition, in further support of sodium channel blockade, the QRS complex widening responded to the administration of sodium bicarbonate.
QRS complex widening can lead to ventricular dysrhythmias, and immediate recognition of this condition is important for pediatricians or emergency physicians. In the case of ingestion, sodium channel blockade is a common mechanism that is treated with high doses of sodium bicarbonate therapy.
Notes
References
1. Köppel C, Tenczer J, Ibe K. Poisoning with over-the-counter doxylamine preparations: an evaluation of 109 cases. Hum Toxicol. 1987; 6:355–9.
2. Derinöz-Güleryüz O. Doxylamine succinate overdose: Slurred speech and visual hallucination. Turk J Pediatr. 2018; 60:439–42.
3. Syed H, Som S, Khan N, Faltas W. Doxylamine toxicity: seizure, rhabdomyolysis and false positive urine drug screen for methadone. BMJ Case Rep. 2009 Mar 17 [Epub]. https://doi.org/10.1136/bcr.09.2008.0879.
4. Carrascosa MF, Caviedes JR, Lucena MI, Cuadrado-Lavín A. Syndrome of inappropriate antidiuresis in doxylamine overdose. BMJ Case Rep. 2012; 2012:bcr-2012-007428.
5. Jo YI, Song JO, Park JH, Koh SY, Lee SM, Seo TH, et al. Risk factors for rhabdomyolysis following doxylamine overdose. Hum Exp Toxicol. 2007; 26:617–21.
6. Lee YD, Lee ST. Acute pancreatitis and acute renal failure complicating doxylamine succinate intoxication. Vet Hum Toxicol. 2002; 44:165–6.
7. Paudel G, Syed M, Kalantre S, Sharma J. Pyrilamine-induced prolonged QT interval in adolescent with drug overdose. Pediatr Emerg Care. 2011; 27:945–7.
8. Wu Chen NB, Schaffer MI, Lin RL, Kurland ML, Donoghue ER Jr, Stein RJ. The general toxicology unknown. II. A case report: doxylamine and pyrilamine intoxication. J Forensic Sci. 1983; 28:398–403.
9. Thakur AC, Aslam AK, Aslam AF, Vasavada BC, Sacchi TJ, Khan IA. QT interval prolongation in diphenhydramine toxicity. Int J Cardiol. 2005; 98:341–3.
Fig. 1.
Initial electrocardiogram after the ingestion of doxylamine, acetaminophen, pyrilamine maleate, caffeine, and fluoxetine. It shows a ventricular rate of 147 beats/minute, QRS duration of 124 milliseconds (reference value, 80-110), QTc interval of 510 milliseconds (350-450), and a complete right bundle branch block.
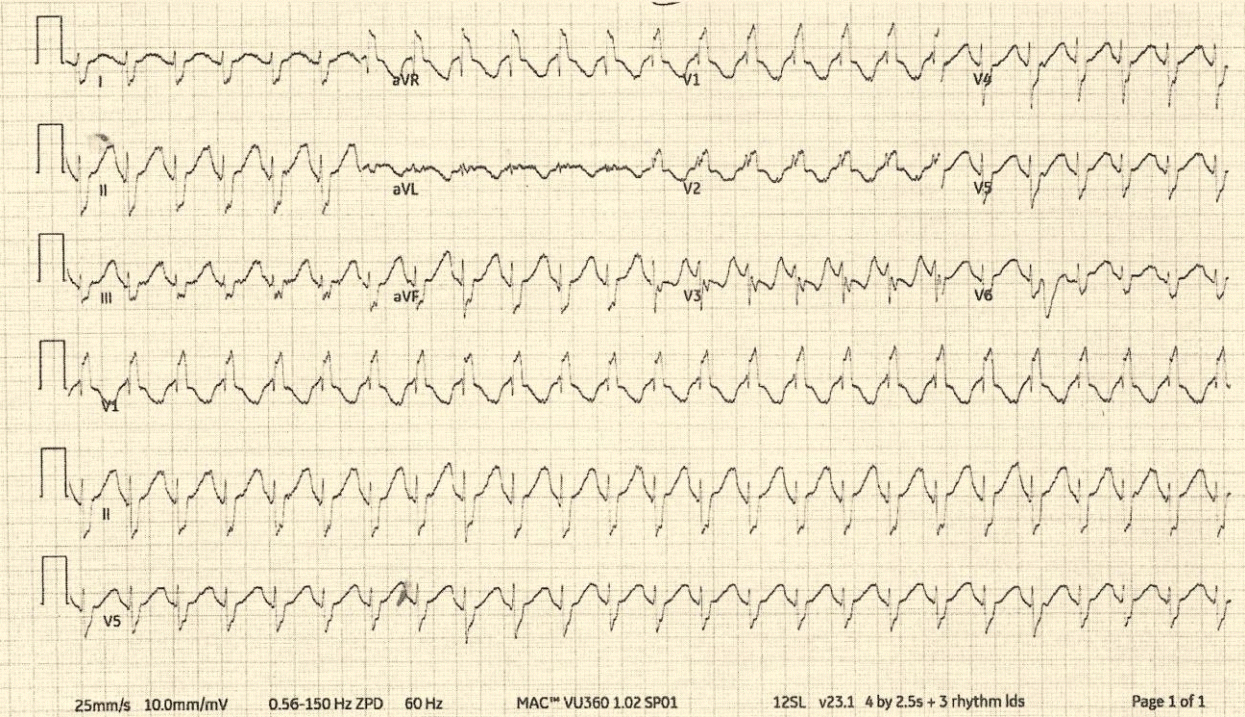
Fig. 2.
Second electrocardiogram obtained 69 minutes after the initial electrocardiogram. It shows a ventricular rate of 131 beats/minute, QRS duration of 96 milliseconds, QTc interval of 608 milliseconds, and an incomplete right bundle branch block.
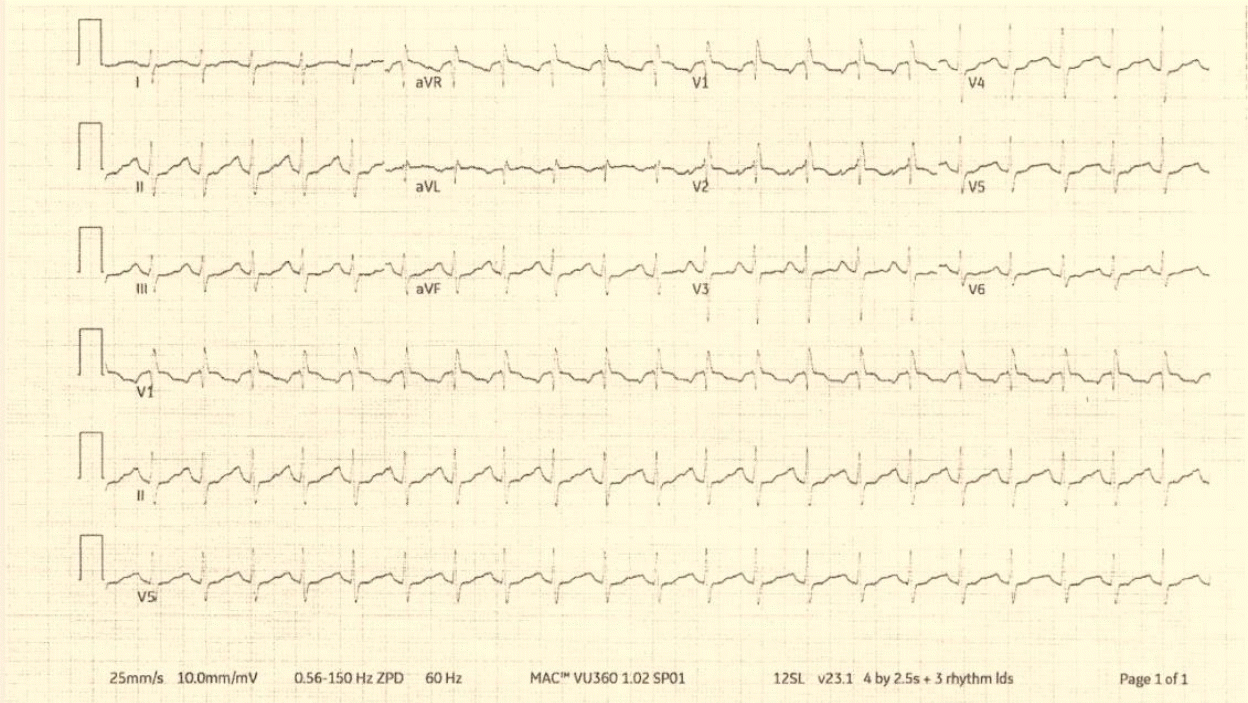
Fig. 3.
The third electrocardiogram obtained 229 minutes after the second electrocardiogram. It shows a ventricular rate of 72 beats/minute, QRS duration of 95 milliseconds, QTc interval of 453 milliseconds, and resolution of the right bundle branch block.
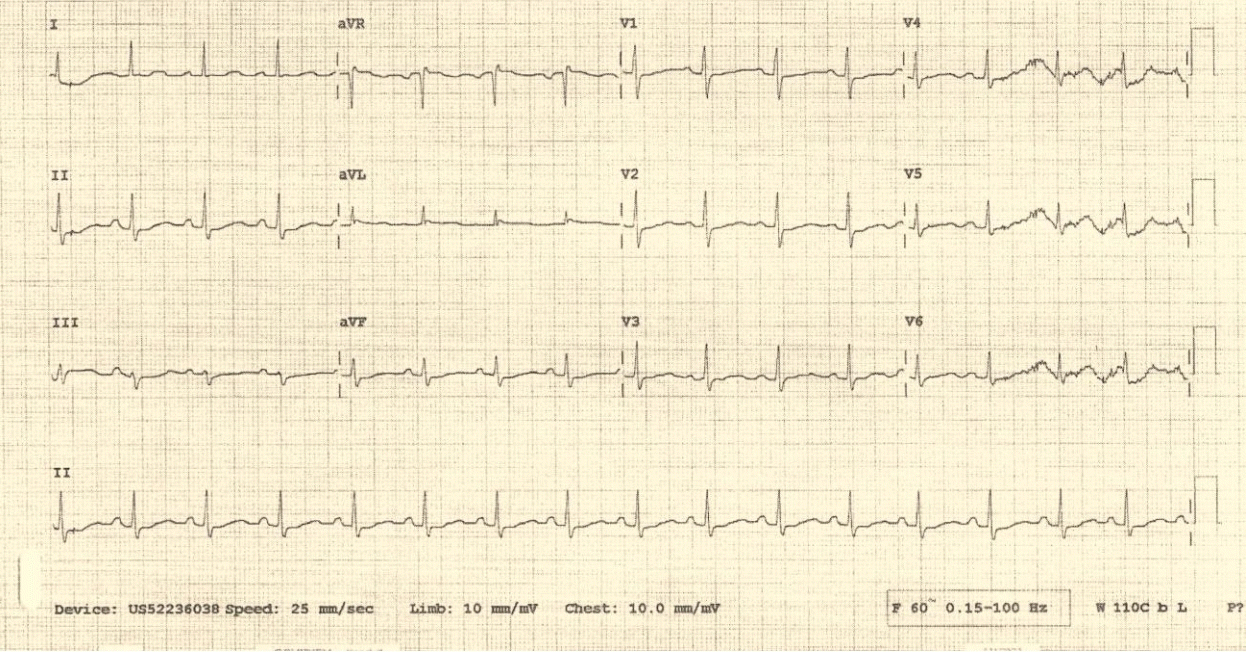
Table 1.
Initial laboratory findings
| Variable | Value (reference value) |
|---|---|
| Complete blood count | |
| White blood cells, ×103/mm3 | 9.3 (5.2-9.7) |
| Hemoglobin, g/dL | 13.8 (11.3-14.7) |
| Platelets, ×103/mm3 | 426 (150-450) |
| Comprehensive metabolic chemistry | |
| Sodium, mmol/L | 136 (134-143) |
| Potassium, mmol/L | 3.1 (3.4-4.7) |
| Chloride, mmol/L | 104 (96-109) |
| Carbon dioxide, mmol/L | 14 (20-31) |
| Anion gap, mmol/L | 18 (< 12) |
| Blood urea nitrogen, mg/dL | 11 (8-21) |
| Creatinine, mg/dL | 1.3 (0.4-0.9) |
| Glucose, mg/dL | 138 (60-105) |
| Calcium, mg/dL | 7.9 (8.9-10.7) |
| Albumin, g/dL | 4.1 (3.7-5.6) |
| Total protein, g/dL | 7.0 (6.3-8.6) |
| Total bilirubin, mg/dL | 0.6 (0.2-1.2) |
| Alkaline phosphatase, U/L | 60 (45-116) |
| Alanine aminotransferase, U/L | 22 (10-35) |
| Aspartate aminotransferase, U/L | 51 (5-30) |
| Acetaminophen, μg/mL | 7.6 (< 5) |
| Salicylates, μg/mL | < 1.7 |
| Ethanol, mg/dL | < 10 |
| Creatine kinase, U/L | 184 (27-140) |
| Urine | |
| Drug screen* | All negative |
| Human chorionic gonadotropin | Negative |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download