Abstract
Purpose
Acetaminophen overdose accounts for the largest proportion of pediatric poisoning reported in South Korea. We investigated the characteristics and prognosis of pediatric acetaminophen overdose.
Methods
We retrospectively reviewed medical records of patients younger than 19 years with acetaminophen overdose who visited the emergency department (ED) of Pusan National University Children’s Hospital from January 2009 through December 2022. We investigated age, sex, dose, clinical findings, and treatment, and compared the differences in the variables according to the intentionality of ingestion and the presence of hepatotoxicity.
Results
Among the 132,691 pediatric patients who visited the ED during the period, 555 did for drug intoxication, of whom 51 with acetaminophen overdose were included in this study. The overdose was intentional in 43 patients (the intentional group; median age, 15.4 years [interquartile range, 13.9-16.8]) and accidental in 8 patients (the accidental group; 7.7 years [2.3-15.4]). The mean ingested dose was higher in the intentional group than in the accidental group (170.3 ± 129.0 vs. 105.3 ± 37.5 mg/kg; P = 0.016). Hospitalization tended to be implemented more frequently in the intentional group (53.5% vs. 12.5%; P = 0.081). All patients with hepatotoxicity (n = 10) belonged to the same group, and showed a higher or longer median age (15.9 [14.6-17.1] vs. 13.8 years [13.7-16.4]; P = 0.030), mean ingested dose (13,230.0 ± 10,544.8 vs. 7,654.0 ± 6,752.3 mg; P = 0.043), mean time from ingestion to arrival at the ED (22.6 ± 23.4 vs. 5.5 ± 6.4 hours; P = 0.048), and frequency of hospitalization (80.0% vs. 39.0%; P = 0.048).
Acetaminophen overdose can lead to hepatotoxicity if an ingested single dose exceeds 120-150 mg/kg in children (1-3). An injury surveillance system based on 20 emergency departments (EDs) operated by the Korean Disease Control and Prevention Agency showed acetaminophen (34.8%), psychotropics (15.1%), and sedative-hypnotics (10.4%) as the 3 most common therapeutic drugs causing intentional poisoning from 2011 through 2016 (4). Since acetaminophen was designated as a home medicine in November 2012 by the Korean government, the drug can be purchased over the counter at convenience stores (5). The Bigdata Open portal provided by the Korean Health Insurance Review and Assessment Service showed an increase in annual incidences of patients younger than 20 years diagnosed with acetaminophen poisoning (Korean Standard Classification of Diseases code T39.1) from January 2018 through December 2022 (6). Similar changes in public access to acetaminophen in Sweden and Ireland have increased the occurrences of poisoning by it (7,8).
From the abovementioned perspective, it is necessary to be aware of the characteristics, treatment, and prognosis of patients with acetaminophen poisoning. The authors aimed to identify the characteristics and outcomes of pediatric patients with acetaminophen overdose who visited the ED.
This study retrospectively included medical records of pediatric patients who visited the ED of Pusan National University Children’s Hospital (PNUCH), located in Yangsan, Korea, from January 2009 through December 2022 due to drug intoxication (Korean Standard Classification of Diseases code T50.9) or acetaminophen poisoning (T39.1). PNUCH runs a pediatric emergency medical center that provides care for children residing in Busan, Ulsan, or Gyeongsangnam-do, and receives approximately 9,300 pediatric patients annually. In this single-center study, we included patients younger than 19 years who were given a code T50.9 or T39.1, and who ingested acetaminophen in excess of the therapeutic dose (> 30 mg/kg or > 1,500 mg in absolute amount as a single dose) or multiple drugs in combination with acetaminophen. We excluded those who ingested the drug within therapeutic doses. This study was approved by the institutional review board of Pusan National University Yangsan Hospital with a waiver for informed consent (IRB no. 05-2020-215).
We administered activated charcoal if acetaminophen was taken within 4 hours of arrival at the ED and if no vomiting was reported. Nasogastric lavage was performed if the drug was taken within 1 hour of the arrival. N-acetylcysteine (NAC) was administered intravenously by the 21-hour protocol within 24 hours of drug ingestion if the dose was > 150 mg/kg or 7.5 g, or when the use was deemed otherwise necessary given the symptoms or elapsed time of ingestion (9). Patients who attempted suicide or self-harm were classified into the intentional group and those who took the dose incorrectly or accidentally without supervision were done into the accidental group. All patients in the former group were recommended psychiatric interviews. In addition, those were further classified according to the presence of hepatotoxicity (hepatotoxicity vs. no hepatotoxicity groups).
Age and weight were based on data from the 2000 Centers for Disease Control and Prevention growth charts (10). Key terms are defined in Appendix 1 (10.22470/pemj.2024.01039). Based on the definitions, we investigated the following variables: age, age groups (0-5, 6-10, and 11-18 years), female sex, intention of ingestion, history of mental illnesses, time from ingestion to arrival at the ED, and co-ingestions. According to the intention, we additionally analyzed ingested dose, history of suicide attempt, multiple ingestion (2 or ≥ 3 drugs), time of ingestion (00:00-05:59, 06:00-11:59, 12:00-17:59, or 18:00-23:59), hepatotoxicity or nephrotoxicity (Appendix 1), treatment (NAC, activated charcoal, or supportive care), and hospitalization.
As per the presence of hepatotoxicity, we further analyzed the patients’ weight and the relevant status (underweight, normal, overweight, or obese [Appendix 1]), and last suicide attempts (tracked until December 2022). In addition, we compared the overall trends of acetaminophen poisoning per year, sex, and age group of the study population with the equivalent values from the Korean Health Insurance Review and Assessment Service data, as representative of the nationwide data (6).
Categorical variables were compared using Pearson’s chi-square tests or Fisher’s exact tests. Statistical significance was set at P < 0.05. Multivariable analysis was conducted using significant factors with a P < 0.05 through the univariate analyses using a logistic regression model. All analyses were performed using R software version 4.3.1 for Windows (R Core Team, 2024).
Of the 132,691 pediatric patients who visited the ED during the study period, 555 (0.4%) experienced poisoning. Among these patients, 51 patients (9.2%) with acetaminophen overdose were enrolled. Their characteristics are summarized in Table 1. The number of patients diagnosed with acetaminophen poisoning from 2010 through 2022 at PNUCH markedly increased over time (Fig. 1A-C). Although the cause is unknown, a similar pattern was confirmed in the other graphs as the number of patients increased from 2018 onwards (Fig. 1D-F).
A median age was older in the intentional group than in the accidental group (15.4 vs. 7.7 years). Except for 1 mentally retarded patient and 2 with headache, all teenagers belong to the intentional group. Girls were more common in the intentional group (86.0% vs. 25.0%). The mean ingested dose was higher in the intentional group (170.3 ± 129.0 vs. 105.3 ± 37.5 mg/kg) (Table 2). All patients in the intentional group underwent a psychiatric interview, and 9 of them were hospitalized in the psychiatric ward after the acute treatment against the poisoning.
Ten patients (19.6%) developed hepatotoxicity while 7 (13.7%) did nephrotoxicity. Among those who developed hepatotoxicity, 7 patients had toxicity at the time of hospitalization, whereas the other 3 showed concentrations of aminotransferases within the normal ranges at the ED but later developed toxicity. A median age was older in the hepatotoxicity group than in the accidental group (15.9 vs. 13.8 years). A mean weight was higher in the former group than that in the counterpart. A mean time from ingestion to arrival at the ED was longer in the hepatitis group. The hepatotoxicity group showed a higher mean dose ingested on a mg/kg basis. than the non-hepatotoxicity group in this study, but it was not significant (Table 3). Likewise, the patients with nephrotoxicity reported a slightly but not significantly higher mean dose than those without nephrotoxicity (165.4 ± 72.3 vs. 159.3 ± 143.3 mg/kg; P = 0.915). No patients had serum creatinine concentrations above the upper normal limit. No meaningful results were found in the multivariable logistic regression, which was performed using significant factors through the univariable analyses.
In the hepatotoxicity group, 8 patients (80.0%) were treated with NAC and/or charcoal; the remaining patients refused the treatment and were discharged against medical advice. All hospitalized patients recovered without developing a concentration of alanine aminotransferase higher than 1,000 IU/mL during hospitalization.
Owing to the easy accessibility to acetaminophen, it is reported as a main cause of pediatric poisoning regardless of intentionality (1,11-13). For the same reason, it is often used as a mean of suicide among Korean adolescents (14). Fig. 1 shows a similarity in the increasing trend between the study population and the Korean nationwide population. In particular, the nationwide data showed that the number of girls and those older than 10 years has suddenly increased since 2018. Considering the age range, the main cause was presumed to be intentional, and further research is required to determine the specific cause.
Several studies have shown that pediatric patients with poisoning are characterized by a bimodal peak age distribution, which consists of accidental overdose under the age of 5 years and intentional overdose in teenagers (11,12). Studies in Singapore and Hong Kong showed that the frequencies of female adolescents were higher in the intentional group, with no differences in gender distribution in the accidental group (12,13). In a single-center study of drug overdose in pediatric suicide attempts, all patients were teenagers with girls being predominant (14). Similarly, in our study, all teenagers except for 3 patients ingested acetaminophen for suicidal or self-harm purposes, and the frequency of girls was significantly higher (P = 0.001).
All 15 patients who ingested acetaminophen during 00:00-05:59 reported their intentions on purpose (P = 0.042). Although the numbers of patients who ingested during 06:00-17:59 and 18:00-23:59 were similar (15 vs. 13), the proportion of patients during 06:00-17:59 was higher in the accidental group.
Hepatotoxicity is a major consequence of acetaminophen poisoning, which can lead to hospitalization or death. A study of 348 pediatric acute liver failure (PALF) patients in North America and the United Kingdom reported by the PALF study group showed acute acetaminophen toxicity as the most common cause of PALF in patients aged 3 years or older (7). The incidence of renal insufficiency is known to be 1%-2% and occurs through a mechanism similar to hepatotoxicity (15). In our study, the occurrence of hepato- and nephro-toxicity was limited to the intentional group.
The frequency of NAC administration was higher in the intentional group than in the accidental group. However, the difference was not statistically significant (P = 0.285). The number of cases requiring hospitalization was significantly higher in the intentional group. All patients were discharged without death or sequelae. According to a study by the PALF study group, the spontaneous recovery rate of acetaminophen-induced PALF was 94%, which was higher than that in patients with non-acetaminophen-induced liver damage (41%) (3). In a study of Schmidt (16) on patients of all ages with acetaminophen poisoning, a patient’s age was an important risk factor for acetaminophen-induced liver damage, and the risk of fulminant liver failure or death was greater, especially in those older than 40 years. This is explained by the fact that younger individuals have a larger functioning liver cell mass and extrahepatic metabolism of toxins that better compensate for acute liver damage than older ones.
As for the limitations of this study, due to the retrospective study design, the heights of some patients were not measured and thus, their body mass indexes could not be calculated. Additionally, serum acetaminophen concentration was not measured per the ED protocol. Although all patients who intentionally ingested were referred for psychiatric interview, some of their parents refused it. In this study, no patients developed a concentration of alanine aminotransferase higher than 1,000 IU/mL, which is commonly cited as a critical hepatotoxicity (17), suggesting that there is a limitation in examining a major trigger of such hepatotoxicity. Finally, the small size of the study population might be related to the lack of significant findings of multivariable analysis.
All patients who visited the ED with acetaminophen overdose were discharged without any physical problems after appropriate treatment. In particular, the intentional group tended to ingest higher doses of acetaminophen, and most were administered NAC or hospitalized. Therefore, patients with intentional acetaminophen poisoning require active treatment, such as activated charcoal or NAC, immediately upon arrival at EDs. Most patients in the intentional group were girls, and 39.5% reported a history of mental illnesses or suicide attempt. Suicidal ingestion is not only a psychiatric emergency but also a risk factor for recurrence, suggesting that active psychiatric treatment is essential.
In conclusion, it is necessary to track the occurrence of hepatotoxicity in patients who intentionally ingest acetaminophen. This article may be meaningful in understanding the characteristics of acetaminophen overdose seen in Korean children or adolescents, and expected to serve as a basis for multicenter research.
Notes
Author contributions
Conceptualization, Methodology, Project administration, Resources, and Validation: YJ Lee and TJ Lim
Data curation and Formal analysis: SJ Sohn
Investigation: SJ Sohn and TJ Lim
Software and Supervision: TJ Lim
Writing-original draft: SJ Sohn
Writing-review and editing: YJ Lee and TJ Lim
All authors read and approved the final manuscript.
References
1. Kanabar DJ. A clinical and safety review of paracetamol and ibuprofen in children. Inflammopharmacology. 2017; 25:1–9.
2. Alander SW, Dowd MD, Bratton SL, Kearns GL. Pediatric acetaminophen overdose: risk factors associated with hepatocellular injury. Arch Pediatr Adolesc Med. 2000; 154:346–50.
3. Squires RH Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006; 148:652–8.
4. Moon J, Chun B, Cho Y, Lee S, Jung E, et al. Characteristics of emergency department presentations of pediatric poisoning between 2011 and 2016: a retrospective observational study in South Korea. Pediatr Emerg Care. 2021; 37:e261–8.
5. Ministry of Health and Welfare (MOHW), Republic of Korea. Home medicine will be sold at convenience stores starting in November this year [Internet]. MOHW; c2024 [cited 2024 Apr 10]. Available from: https://www.mohw.go.kr/board.es?mid=a10503000000&bid=0027&act=view&list_no=271028&tag=&nPage=1. Korean.
6. Big Data Strategy Department, Health Insurance Review and Assessment Service (HIRA). Disease subclassification (4th level corporal) statistics [Internet]. HIRA; c2023 [cited 2024 Apr 10]. Available from: https://opendata.hira.or.kr/op/opc/olap4thDsInfoTab2.do. Korean.
7. Gedeborg R, Svennblad B, Holm L, Sjogren H, Bardage C, Personne M, et al. Increased availability of paracetamol in Sweden and incidence of paracetamol poisoning: using laboratory data to increase validity of a population-based registry study. Pharmacoepidemiol Drug Saf. 2017; 26:518–27.
8. O'Rourke M, Garland MR, McCormick PA. Ease of access is a principal factor in the frequency of paracetamol overdose. Ir J Med Sci. 2002; 171:148–50.
9. Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977; 2:432–4.
10. U.S. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. CDC growth charts [Internet]. U.S. Department of Health & Human Services; c2024 [cited 2024 Feb 15]. Available from: https://www.cdc.gov/growthcharts/cdc_charts.htm.
11. Mintegi S, Azkunaga B, Prego J, Qureshi N, Dalziel SR, Arana-Arri E, et al. International epidemiological differences in acute poisonings in pediatric emergency departments. Pediatr Emerg Care. 2019; 35:50–7.
12. Koh SH, Tan KHB, Ganapathy S. Epidemiology of paediatric poisoning presenting to a children's emergency department in Singapore over a five-year period. Singapore Med J. 2018; 59:247–50.
13. Hon KL, Ho JK, Leung TF, Wong Y, Nelson EA, Fok TF. Review of children hospitalised for ingestion and poisoning at a tertiary centre. Ann Acad Med Singap. 2005; 34:356–61.
14. Kweon YS, Hwang S, Yeon B, Choi KH, Oh Y, Lee HK, et al. Characteristics of drug overdose in young suicide attempters. Clin Psychopharmacol Neurosci. 2012; 10:180–4.
15. Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol. 2008; 4:2–6.
16. Schmidt LE. Age and paracetamol self-poisoning. Gut. 2005; 54:686–90.
17. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988; 319:1557–62.
Fig. 1.
Figures by year, age group, and sex of patients who visited the emergency department with acetaminophen overdose from 2010 through 2022. The numbers of patients are plotted per the sex (A) and age group (B and C). These trends shows similar patterns with the equivalent values based on the Korean Health Insurance Review and Assessment Service data (D-F).
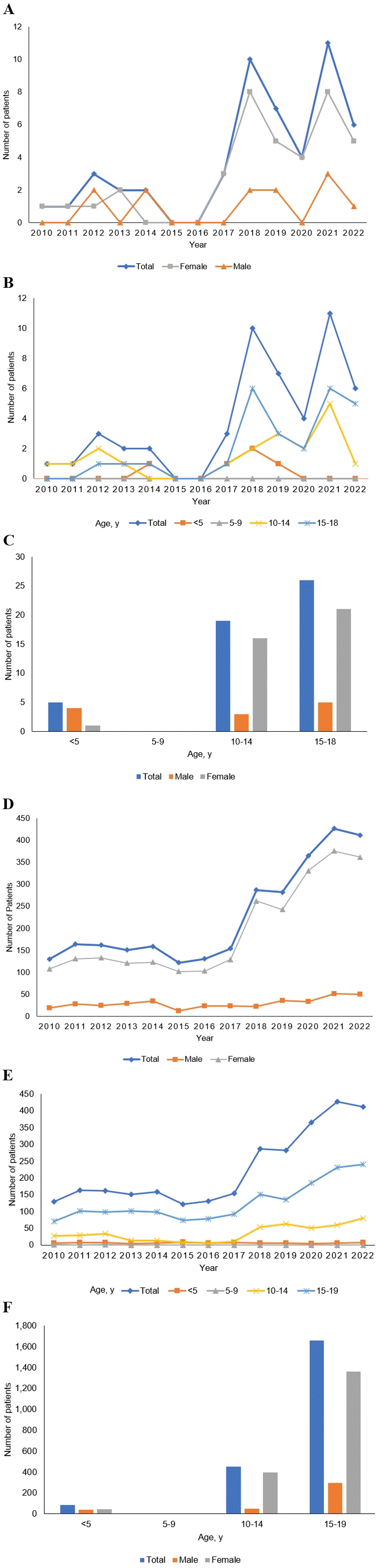
Table 1.
Characteristics of pediatric acetaminophen overdose (N = 51)
| Characteristics | Data | |
|---|---|---|
| Age, y | 15.0 (13.7-16.7) | |
| Age group, y | ||
| 0-5 | 5 (9.8) | |
| 6-10 | 0 (0) | |
| 11-18 | 46 (90.2) | |
| Girls | 39 (76.5) | |
| Intentional ingestion | 43 (84.3) | |
| History of mental illnesses | 17 (33.3) | |
| Time ingestion-arrival, h | ||
| 0-4 | 25 (49.0) | |
| 5-8 | 11 (21.6) | |
| > 8 | 15 (29.4) | |
| Substance of co-ingestion* | ||
| Other antipyretics | 4 (14.8)† | |
| Psychotropic | 10 (37.0)† | |
| Hypnotics | 4 (14.8)† | |
| Cold medication | 4 (14.8)† | |
| Others‡ | 5 (18.5)† |
Table 2.
Clinical and therapeutic characteristics of the patients according to the intention of ingestion
Table 3.
Clinical and therapeutic characteristics of the patients according to the status of hepatotoxicity
| Hepatotoxicity (N = 10) | No hepatotoxicity (N = 41) | P value | |
|---|---|---|---|
| Age, y | 15.9 (14.6-17.1) | 13.8 (13.7-16.4) | 0.030 |
| Age group, y | 0.569 | ||
| 0-5 | 0 (0) | 5 (12.2) | |
| 6-10 | 0 (0) | 0 (0) | |
| 11-18 | 10 (100) | 36 (87.8) | |
| Girls | 8 (80.0) | 31 (75.6) | > 0.999 |
| Weight, kg | 69.2 ± 27.1 | 53.1 ± 18.3 | 0.038 |
| Dose ingested, mg/kg | 222.1 ± 174.2 | 145.9 ± 124.8 | 0.112 |
| Dose ingested, mg | 13,230.0 ± 10,544.8 | 7,654.0 ± 6,752.3 | 0.043 |
| Time ingestion-arrival, h | 22.6 ± 23.4 | 5.5 ± 6.4 | 0.048 |
| Weight for age | 0.066 | ||
| Underweight | 1 (10.0) | 0 (0) | |
| Normal | 5 (50.0) | 31 (75.6) | |
| Overweight | 2 (20.0) | 8 (19.5) | |
| Obese | 2 (20.0) | 2 (4.9) | |
| Body mass index* | 0.762 | ||
| Underweight | 1 (12.5) | 3 (10.0) | |
| Normal | 4 (50.0) | 18 (60.0) | |
| Overweight | 0 (0) | 2 (6.7) | |
| Obese | 3 (37.5) | 7 (23.3) | |
| History of mental illnesses | 2 (20.0) | 15 (36.6) | 0.533 |
| History of suicide attempt | 5 (50.0) | 12 (29.3) | 0.383 |
| Multiple ingestion | 2 (20.0) | 20 (48.8) | 0.196 |
| Use of NAC or charcoal | 8 (80.0) | 34 (82.9) | 0.483 |
| Hospitalization | 8 (80.0) | 16 (39.0) | 0.048 |
| Later suicide attempts† | 1 (10.0) | 4 (9.8) | > 0.999 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download