Abstract
A 24-year-old male was admitted with progressive cervical hypesthesia, tetraparesis, dyspnea, and a history of craniofacial fracture. Spinal magnetic resonance imaging (MRI) showed brainstem edema extending to the thoracic spine with multiple prominent perimedullary vascular structures. Cerebral digital-substraction angiography revealed Barrow type A carotid-cavernous fistula. Total occlusion with preservation of internal carotid artery flow was achieved using 1 detachable balloon and 6 coils. Postoperatively, immediate respiratory recovery, gradual extremities strength improvement, and right abducens nerve palsy were found. One month follow-up cervical MRI showed good recovery of spinal cord edema and perimedullary veins.
Carotid-cavernous fistula (CCF) is an arteriovenous shunt inside the cavernous sinus (CS) [1], contributing to 2.5–3% of all intracranial vascular pathology and most commonly caused by trauma, followed by rupture of internal carotid artery (ICA) aneurysm in CS, connective tissue disease (e.g., Ehlers–Danlos syndrome, fibromuscular dysplasia), and iatrogenic [2,3]. Most CCFs, due to drainage to ophthalmic veins, cause severe congestion in the CS so that ocular symptoms are commonly found in high-flow type [4]. Only 7 cases of CCF with myelopathy due to perimedullary venous drainage have been reported [5-10]. This paper discusses a case of CCF after head injury in which delayed myelopathic symptoms manifested.
A 24-year-old male was referred to our hospital with progressive cervical hypesthesia and tetraparesis since a week ago. The tetraparesis worsen from a muscle strength of 4 to 1 and was accompanied with dyspnea, which required a supplemental oxygen mask (10 L/min). He presented with a history of head injury with left craniofacial fracture that needed repair 3 years prior without any neurological deficit. Chemosis, diplopia, and proptosis were not found. Spinal magnetic resonance imaging (MRI) was done and showed edema in the brainstem with extension to the thoracic spine with multiple prominent perimedullary vascular structures (Fig. 1). Spinal dural arteriovenous fistula was suspected. Cerebral digital subtraction angiography (DSA) revealed right high-flow CCF (Barrow type A) with aneurysms and retrograde flow to the posterior draining of CS and reflux to perimedullary and peripontomesencephalic veins. DSA showed no ophthalmic vein filling and any fistula between external carotid artery branches with CS. This finding explained progressive myelopathic symptoms that occurred in this patient was due to venous hypertension. A balloon occlusion test was not performed in this case because of the angio-structure and drainage pattern, so the primary purpose was to close the fistula canal and preserve ICA flow.
Our treatment strategy for this case was to close the fistula orifice by detachable balloon and coils if needed. Before endovascular therapy was performed, 75 mg clopidogrel was given once daily for 5 days. After general anesthesia, the patient underwent endovascular therapy via femoral artery puncture, and heparin saline was given continuously of 1,000 U/hour during the procedure. First, a 6-French Fargomax Guiding catheter (Balt Extrusion SAS) was placed in the right ICA around the petrous section. Then a Magic microcatheter for a detachable balloon (Balt Extrusion SAS) was guided inside the CS close to the fistula orifice using a hybrid wire guiding catheter (Balt Extrusion SAS). A Goldbal2 was detached inside the CS, but it only allowed partial closure of the fistula orifice. After removing the Magic microcatheter, a Vasco+ 10 MP (Balt Extrusion SAS) was guided and placed behind the Goldbal2. Six coils (Optima Complex-10 Soft; Balt Extrusion SAS) were used to occlude the flow from the ICA. Thereafter, immediate angiography verified total occlusion of the fistula without any more venous drainage and well-preserved ICA flow.
Immediately after treatment, respiratory function recovered followed by gradual improvement of extremities strength, but right abducens nerve palsy was found. The patient regained the ability to stand and walk slowly after 1 month rehabilitation. Cervical spinal MRI scan after 1 month of treatment showed recovery of spinal cord edema and disappearance of the perimedullary veins. The patient was planned to undergo outpatient physical therapy for 3 months.
CCF can be caused by trauma in around 0.2–4% cases, with a 3.8% mortality rate if left untreated [11,12]. The pathogenesis of traumatic CCF is laceration of the ICA wall due to ripping forces during trauma or skull base fracture that forms an CSICA fistula, enabling direct flow and increasing intraluminal pressure that leads to increased interstitial and intracellular edema. This causes malfunction of neurons in the target region, and explains the signs and symptoms encountered [11]. Clinical signs and symptoms are determined by their drainage and flow pattern, which is best shown by digital substraction angiography [8,13].
The clinical signs of cervical myelopathy are tetraparesis, hypesthesia, and dyspnea, which is rarely caused by CCF [8]. Alternatively, a dural arteriovenous fistula that drains into perimedullary veins and causes myelopathy is quite common [14,15]. To the best of the authors’ knowledge, only 7 cases of CCF with symptoms of progressive myelopathy been reported. Four of the 7 reported CCF cases were traumatic, and the others were spontaneous [5-10].
Symptoms and clinical signs mostly occurred in the short term after head trauma. The longest onset published was 5 years for anterior drainage and 2 years for posterior drainage [8]. CS consists of some venous compartments that are connected by some anastomoses. Therefore, delayed ocular signs and symptoms in traumatic CCF can occur if there is a low-flow direct orifice fistula located in the lateral space inside the CS, which has a little chamber with minor contact with others chambers and is not accompanied with steal phenomena [16]. The delayed clinical sign in this case is similar, with a traumatic CCF with a delayed onset of myelopathy 5 years after head injury. The same delayed clinical presentations was caused by the location of an orifice fistula in the lateral space of CS that was connected to another compartment through a narrow and low-flow shunt. Based on the onset of the clinical presentation, this case can be considered as direct CCF with low-flow [8]. The CCF that occurred on the contralateral side of the fracture was also unusual. It might be caused by escalation of venous sinus pressure due to a microtear in the CS vessels [11,17].
Endovascular therapy is the first option for CCF with a 90–100% recovery rate and minimal complications [3,11,18]. Selected treatments for the reported cases were 1 case with surgical ligation, 1 case with a combination of glue embolization and a stereotactic gamma-knife, 4 cases with coil embolization, and 1 case with combination coils and Onyx liquid embolization (Table 1). Several complications of endovascular therapy have been reported including parent artery occlusion, cerebral ischemia, diabetes insipidus, thrombosis in femoral vein, and cranial nerve palsies, with a range 2–16.7%. The most common complications are 3rd, 4th, and 6th nerve palsies; however, around 67% of patients regain complete recovery [19-21]. In this case, the patient experienced right abducens nerve palsy that might be caused by the coils mass effect in the lateral space of the CS and a compressed abducens nerve [20]. The prognosis after treatment is usually good with recovery within hours to weeks. Recurrence of CCF is rare and can be treated by embolization [3,11]. From 4 reported cases, 3 cases regained recovery within months and only 1 case demonstrated a persistent symptom [5-8].
In conclusion, CCFs have heterogenous clinical manifestations, and an uncommon one is myelopathy. Patients with a history of head injury, especially with craniofacial fractures, are recommended for at least 3 years of follow-up. This is to ensure that CCF or any other complication due to trauma can be detected early and promptly treated to reduce morbidity and mortality and maximize prognosis.
Notes
Ethics Statement
Informed consent was obtained from the patient for participation to this study and publication of all their data and/or images included. This study waived approval of the Institutional Review Board (IRB).
Author Contributions
Concept and design: ST, HH, and HS. Analysis and interpretation: ST, HH, AMS, HS, and AJ. Data collection: ST, AMS, HS, and AJ. Writing the article: ST, HS, and AJ. Critical revision of the article: ST, HH, AMS, HS, and AJ. Final approval of the article: ST. Statistical analysis: ST. Overall responsibility: ST.
REFERENCES
1. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985; 62:248–256.
2. Xu XQ, Liu S, Zu QQ, Zhao LB, Xia JG, Zhou CG, et al. Follow-up of 58 traumatic carotid-cavernous fistulas after endovascular detachable-balloon embolization at a single center. J Clin Neurol. 2013; 9:83–90.
3. Henderson AD, Miller NR. Carotid-cavernous fistula: current concepts in aetiology, investigation, and management. Eye (Lond). 2018; 32:164–172.
4. Korkmazer B, Kocak B, Tureci E, Islak C, Kocer N, Kizilkilic O. Endovascular treatment of carotid cavernous sinus fistula: a systematic review. World J Radiol. 2013; 5:143–155.
5. Narita Y, Watanabe Y, Hoshino T, Okada M, Yamamoto Y, Kuzuhara S. Myelopathy due to large veins draining recurrent spontaneous caroticocavernous fistula. Neuroradiology. 1992; 34:433–435.
6. Ko SB, Kim CK, Lee SH, Yoon BW. Carotid cavernous fistula with cervical myelopathy. J Clin Neurosci. 2009; 16:1350–1353.
7. Herrera DA, Vargas SA, Dublin AB. Traumatic carotid-cavernous fistula with pontomesencephalic and cervical cord venous drainage presenting as tetraparesis. J Neuroimaging. 2011; 21:73–75.
8. Ding CL, Zhang CL, Hua F, Xi SD, Zhou QW, Wang HJ, et al. Traumatic carotid-cavernous fistula with perimedullary venous drainage and delayed myelopathy: A case report. Med Int (Lond). 2021; 1:16.
9. Ract I, Drier A, Leclercq D, Sourour N, Gabrieli J, Yger M, et al. Extensive basal ganglia edema caused by a traumatic carotid-cavernous fistula: a rare presentation related to a basal vein of Rosenthal anatomical variation. J Neurosurg. 2014; 121:63–66.
10. Ma YH, Shang R, Lin S, Li SH, Wang T, Zhang CW. Case report: delayed quadriplegia from traumatic carotid cavernous fistula: a rare case with perimedullary venous drainage. Front Neurol. 2023; 14:1224425.
11. Ellis JA, Goldstein H, Connolly ES Jr, Meyers PM. Carotid-cavernous fistulas. Neurosurg Focus. 2012; 32:E9.
12. Ying Sze C, Bakin S, Ismail R. Neuropsychiatric presentation in indirect carotid-cavernous fistula: a case report. Cureus. 2023; 15:e37523.
13. Aralasmak A, Karaali K, Cevikol C, Senol U, Sindel T, Toprak H, et al. Venous drainage patterns in carotid cavernous fistulas. ISRN Radiol. 2014; 2014:760267.
14. Naylor RM, Topinka B, Rinaldo L, Jacobi J, Neth B, Flemming KD, et al. Progressive myelopathy from a craniocervical junction dural arteriovenous fistula. Stroke. 2021; 52:e278–e281.
15. Kim WY, Kim JB, Nam TK, Kim YB, Park SW. Cervical myelopathy caused by intracranial dural arteriovenous fistula. Korean J Spine. 2016; 13:67–70.
16. D’Angelo L, Paglia F, Caporlingua A, Sampirisi L, Guidetti G, Santoro A. Atypical manifestation of direct low-flow carotid-cavernous fistula: case report and review of the literature. World Neurosurg. 2019; 125:456–460.
17. Shim HS, Kang KJ, Choi HJ, Jeong YJ, Byeon JH. Delayed contralateral traumatic carotid cavernous fistula after craniomaxillofacial fractures. Arch Craniofac Surg. 2019; 20:44–47.
18. Ide S, Kiyosue H, Tokuyama K, Hori Y, Sagara Y, Kubo T. Direct carotid cavernous fistulas. J Neuroendovasc Ther. 2020; 14:583–592.
19. Cunningham EJ, Albani B, Masaryk TJ, Rasmussen PA. Temporary balloon occlusion of the cavernous carotid artery for removal of an orbital and intracranial foreign body: case report. Neurosurgery. 2004; 55:1225.
20. Fukawa N, Nakagawa N, Tsuji K, Yoshioka H, Furukawa K, Nagatsuka K, et al. Transarterial and transvenous coil embolization of direct carotid-cavernous fistulas. J Neuroendovasc Ther. 2022; 16:127–134.
21. Yu J, Guo Y, Zhao S, Xu K. Brainstem edema caused by traumatic carotid-cavernous fistula: a case report and review of the literature. Exp Ther Med. 2015; 10:445–450.
Fig. 1.
(A) T2 Sagittal cervical magnetic resonance imaging (MRI): edema of medulla oblongata and cervicothoracic spinal cord and serpentine like signal void structure (suggesting peri medullary vein enlargement) (white arrows). (B) Right internal carotid artery (right anterior oblique view): abnormal filling of the cavernous sinus (CS, black arrow) with an associated aneurysm consistent with direct carotid-cavernous fistula. No anterior drainage, prominent reflux into the superior petrosal sinus (black arrowheads) - inferior petrosal sinus (empty arrow) and peri medullary veins noted. (C) Goldbal2 detached inside the CS. (D) After detaching 3 detachable coils - not fully occluded fistula. (E) Lateral view after adding another 3 detachable coils. (F) Cervical spinal MRI scan after 1 month of treatment showing recovery of spinal cord edema and disappearance of the perimedullary veins.
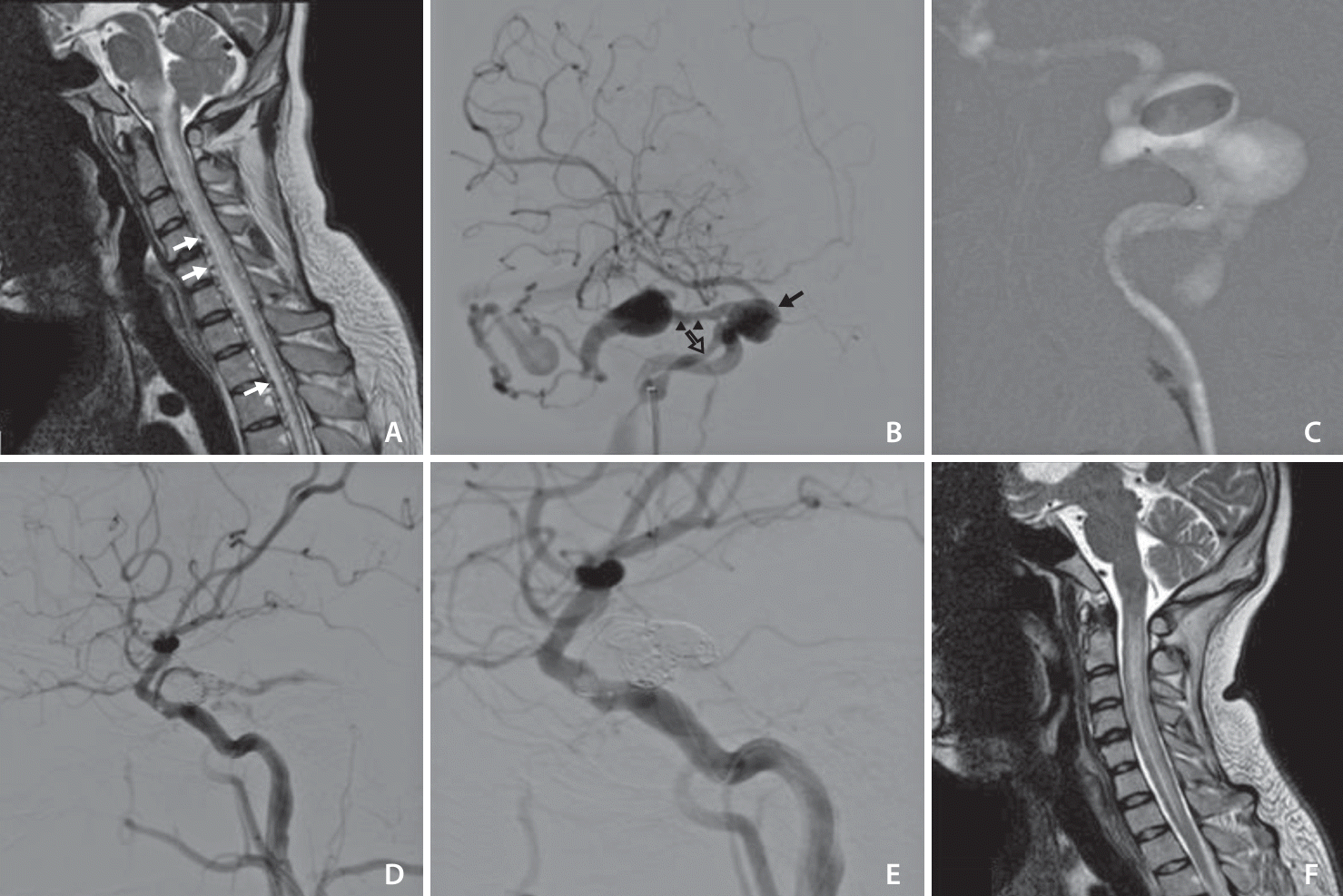
Table 1.
Seven cases of traumatic carotid-cavernous fistula with myelopathy
| Author | Age/sex | Clinical findings | Radiographic findings | Treatment | Follow-up |
|---|---|---|---|---|---|
| Narita et al. [5] | 45/F | Gradual worsening right hemiparesis after CCF surgical closure | Barrow type A CCF | Open surgical ligation | 2 months after operation (R) |
| Ko et al. [6] | 54/M | Slowly progressive quadriparesis | Barrow type D CCF | Glue embolization (n-butyl-2-cyanoacrylate)+stereotactic gamma-knife | 10-month after surgery (R) |
| Herrera et al. [7] | 27/M | Progressive ascending myelopathy, tetraparesis and respiratory insufficiency. history of trauma (+) | Barrow type A CCF | Coil (19 hydrocoilds) embolization of fistula | 4 months after treatment (R) |
| Ding et al. [8] | 45/M | Progressive gait disturbance in left lower limb. history of trauma (+) | Barrow type A CCF | 3 coils and an Onyx liquid embolic system | 6 months after treatment (R) |
| Yu et al. [21] | 51/M | Right exophthalmos and intracranial bruit for 1 week, left hemiparesis, history of trauma (+) | Barrow type A CCF | Transarterial balloon embolization | 6-month follow-up (R) |
| Ract et al. [9] | 45/W | Left hemiparesis, dysarthria, and a comatose state, history of trauma (+) | Barrow type A CCF | Transarterial coiling embolization | 1-month follow-up (R) |
| Ma et al. [10] | 29/M | Sudden onset of headache and quadriplegia, history of trauma (+) | Barrow type A CCF | Transarterial coiling embolization | 6-month follow-up (R) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download