Abstract
The present report describes a patient with spinal cord arteriovenous malformation (AVM) and an associated anterior spinal artery aneurysm presenting with subarachnoid hemorrhage. Diagnostic spinal angiography revealed an intramedullary AVM, located at the T10–T11 level, and a prenidal saccular aneurysm at the junction of the radiculomedullary artery and the anterior spinal axis, fed by the right T8 segmental artery. The patient underwent successful selective coil embolization of the aneurysm. Follow-up angiography 3 months postoperatively showed no recurrence of the aneurysm.
Spinal artery aneurysms are commonly flow-related lesions and are found in association with spinal arteriovenous malformations (AVMs) [1,2]. Prenidal or intranidal aneurysms are related to poor prognosis because of the high incidence of bleeding [1,3,4]. Therefore, most authors agree that associated arterial aneurysms should be prioritized, especially when accounting for subarachnoid hemorrhage (SAH) [3,4]. Because of the significant role of the anterior spinal artery (ASA) in spinal cord vascularization, the endovascular and microsurgical management of an aneurysm located on the anterior spinal axis is challenging [3,4].
We present a case of a patient with a thoracic spinal intramedullary AVM associated with a prenidal ASA aneurysm who presented with SAH. The patient underwent successful selective coil embolization of the aneurysmal sac.
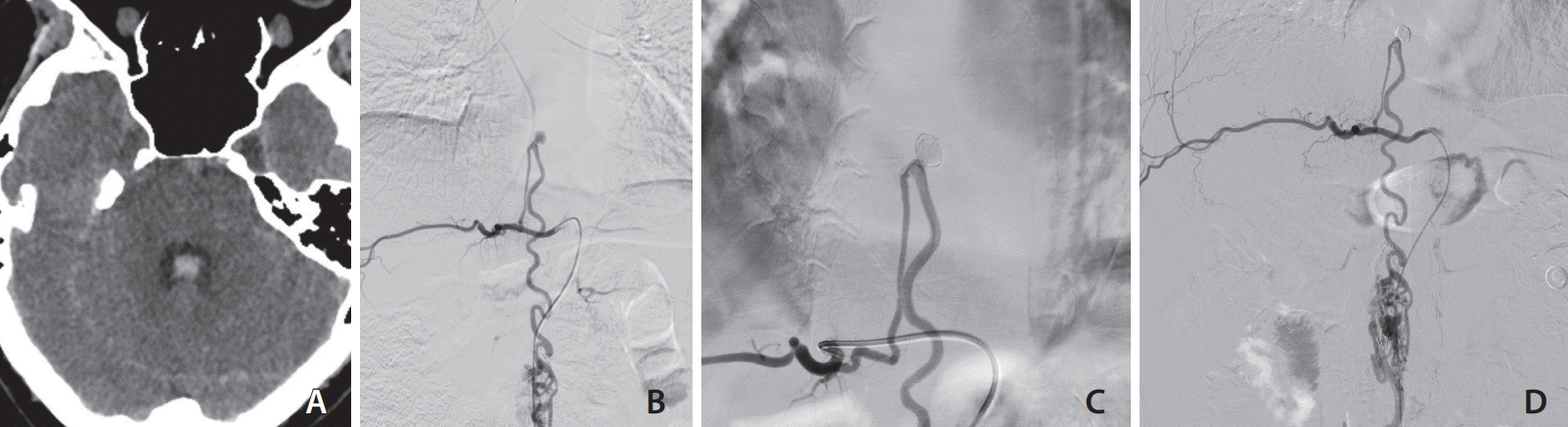
A 37-year-old female patient, with a history of an operated thoracic spinal cord vascular lesion 28 years ago, was admitted to our hospital with acute headache, vomiting, and an episode of loss of consciousness. On admission, the patient had a Glasgow Coma Scale score of 15/15, and according to the Oxford Scale, she had 3/5 muscle strength in the central muscles of the right lower extremity, 2/5 on peripheral muscles of the right lower extremity, and 4/5 on all muscles of the left lower extremity. On upper extremities, the patient had 5/5 on all muscle groups. A brain computed tomography (CT) scan revealed SAH with intraventricular (IV) extension (Fig. 1A). A brain CT angiography was negative. Digital subtraction angiography (DSA) of the brain was normal. Considering the pattern of bleeding (IV extension) and the patient’s history of thoracic spinal cord vascular lesion operation, we decided to perform a spinal DSA. An intramedullary AVM, at the T10–T11 level, supplied mostly by the ASA was demonstrated (Fig. 1B). Superselective catheterization of the right T8 segmental artery showed a dilated radiculomedullary artery, which contributed to the supply of the AVM, and a flow-related, prenidal aneurysm, measuring 1.2 × 1.1 cm, at the junction of the radiculomedullary artery and thoracic anterior spinal axis (Fig. 1B).
Under general anesthesia, the patient underwent endovascular selective coil embolization of the aneurysm. A 5F, C1 Cobra catheter (Merit Medical Systems) was used to catheterize the right T8 segmental artery. Distal catheterization was achieved just proximal to the origin of the radicular artery for better stability. A Headway Duo microcatheter (MicroVention) was then used with a Hybrid 0.008” microwire (Balt) to catheterize the radicular artery and the aneurysmal sac. Five detachable coils were used, and complete aneurysm occlusion was achieved (Fig. 1C). The procedure was uneventful.
At the end of the procedure, the aneurysm was completely occluded and the anterior spinal axis was patent (Fig. 1C). Postoperatively, the patient remained neurologically unchanged. Follow-up angiogram 6 months post embolization showed no recurrence of the aneurysm (Fig. 1D), and the patient was neurologically stable.
Spinal aneurysms (SAs) are rare neurovascular pathologies, with an unclear natural history and management strategy. The incidence of SAs has been significantly lower, accounting for 1 SA every 3,000 spinal angiograms [5]. Madhugiri et al. [6] in their review of the published literature found 140 SAs. They occur in almost equal frequency in males and females, while 10% were seen in children younger than 10 years old.
SAs are typically associated with high-flow conditions such as spinal AVMs, aortic coarctation, or bilateral vertebral artery occlusion, or conditions affecting the spinal vasculature including inflammatory, connective tissue disease, infections, post-transplantation, and trauma [7]. SAs with no identifiable underlying disease are extremely rare and are referred to as isolated [7].
Regarding clinical presentation, SAs are usually detected after they rupture and cause spinal or intracranial SAH. Spinal vascular lesions such as aneurysms account for less than 1% of SAH cases [8]. Less frequently, SAs may be detected incidentally or as a result of compressive myelopathy.
Madhugiri et al. [6] proposed a classification of SAs based on clinical patterns and management options. Type 1 comprises SAs associated with a spinal AVM or arteriovenous fistula and Type 2 comprises isolated SAs. Jung et al. [9] classified the AVM-associated aneurysms into 3 types, based on their location in relation to the nidus: prenidal (arterial), nidal, or postnidal (venous).
There is not enough evidence about prenidal SAs associated with AVMs to draw definitive conclusions about their natural history and treatment benefits. Nonetheless, most authors are convinced that associated SAs, especially in the presence of SAH, should be treated [3,4].
The treatment strategy for SAs associated with AVMs should consider the morphology and the accessibility of the aneurysm, as well as the need to preserve the patency of the parent vessel. Most spinal artery aneurysms are fusiform, so selective coil embolization is not an option. Additionally, aneurysms located in the anterior spinal axis pose a significant challenge because occlusion of the parent artery could potentially be devastating, and therefore are not amenable to parent artery occlusion. Eight papers reported on the treatment of prenidal SAs associated with AVMs [4,10-16]. Four of the reported patients exhibited signs of SAH [4,10,11,16], while 3 patients presented with an aneurysm located on the ASA [4,10,11]. Only 2 patients underwent selective endosaccular coil embolization of the lesion [4,11].
However, in our case, the saccular shape of the ASA aneurysm and its relatively small neck made selective coil embolization feasible. We used coils to effectively control the neck of the aneurysm and avoid occluding the anterior spinal axis. Due to the larger size of the radiculomedullary artery and the ASA caused the high-flow state, allowed safer catheterization and selective coil embolization. Other endovascular techniques that have been reported for SAs, associated with spinal cord AVMs, include glue or particle embolization. Surgical treatment of SAs, with preservation of the parent artery, has also been reported [12-15].
This case highlights the successful selective coil embolization of a prenidal ASA aneurysm associated with a spinal AVM, following SAH. Despite the challenges posed by the aneurysm’s location on the anterior spinal axis, careful consideration of aneurysm morphology and vascular access enabled safe treatment.
Notes
REFERENCES
1. Biondi A, Merland JJ, Hodes JE, Pruvo JP, Reizine D. Aneurysms of spinal arteries associated with intramedullary arteriovenous malformations. I. Angiographic and clinical aspects. AJNR Am J Neuroradiol. 1992; 13(3):913–922.
2. Chen CC, Bellon RJ, Ogilvy CS, Putman CM. Aneurysms of the lateral spinal artery: report of two cases. Neurosurgery. 2001; 48:949–953. discussion 953-954.
3. Konan AV, Raymond J, Roy D. Transarterial embolization of aneurysms associated with spinal cord arteriovenous malformations. Report of four cases. J Neurosurg. 1999; 90(1 Suppl):148–154.
4. Lavoie P, Raymond J, Roy D, Guilbert F, Weill A. Selective treatment of an anterior spinal artery aneurysm with endosaccular coil therapy. Case report. J Neurosurg Spine. 2007; 6:460–464.
5. Djindjian R. Angiography of the spinal cord. Surg Neurol. 1974; 2:179–185.
6. Madhugiri VS, Ambekar S, Roopesh Kumar VR, Sasidharan GM, Nanda A. Spinal aneurysms: clinicoradiological features and management paradigms. J Neurosurg Spine. 2013; 19:34–48.
7. Abdalkader M, Samuelsen BT, Moore JM, Cervantes-Arslanian A, Ong CJ, Setty BN, et al. Ruptured spinal aneurysms: diagnosis and management paradigms. World Neurosurg. 2021; 146:e368–e377.
8. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007; 369:306–318.
9. Jung SC, Song Y, Cho SH, Kim J, Noh SY, Lee SH, et al. Endovascular management of aneurysms associated with spinal arteriovenous malformations. J Neurointerv Surg. 2018; 10:198–203.
10. Baldassarre B, Balestrino A, D’Andrea A, Anania P, Ceraudo M, Truffelli M, et al. Management of spinal aneurysms associated with arteriovenous malformations: systematic literature review and illustrative case. Eur Spine J. 2021; 30:2767–2774.
11. Yamazaki D, Hanaoka Y, Koyama JI, Suzuki Y, Agata M, Abe D, et al. Intraspinal canal platform system for coil embolization of anterior spinal artery aneurysm associated with spinal cord arteriovenous malformation: a case report and literature review. Br J Neurosurg. 2023; 37:1786–1791.
12. Ren Y, He M, You C, Li J. Successful surgical resection of spinal artery aneurysms: report of 3 cases. World Neurosurg. 2018; 109:171–178.
13. Scoville WB. Intramedullary arteriovenous aneurysm of the spinal cord; case report with operative removal from the conus medullaris. J Neurosurg. 1948; 5:307–312.
14. Binder B, Eng GD, Milhorat TH, Galioto F. Spinal arteriovenous malformations in an infant: unusual symptomology and pathology. Dev Med Child Neurol. 1982; 24:380–385.
15. Zhang HJ, Silva N, Solli E, Ayala AC, Tomycz L, Christie C, et al. Treatment options and long-term outcomes in pediatric spinal cord vascular malformations: a case report and review of the literature. Childs Nerv Syst. 2020; 36:3147–3152.
16. Caroscio JT, Brannan T, Budabin M, Huang YP, Yahr MD. Subarachnoid hemorrhage secondary to spinal arteriovenous malformation and aneurysm. Report of a case and review of the literature. Arch Neurol. 1980; 37:101–103.
Fig. 1.
Cerebral computed tomography scan on admission showed subarachnoid hemorrhage with intraventricular extension (A). Spinal cord digital subtraction angiography showed (B) an intramedullary arteriovenous malformation, at the T10–T11 level, supplied mostly by the anterior spinal artery. Immediate post-embolization angiography (C) showed complete occlusion of the aneurysm. Follow-up angiogram (D) 6 months post embolization showed no recurrence of the aneurysm.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download