INTRODUCTION
CASE REPORT
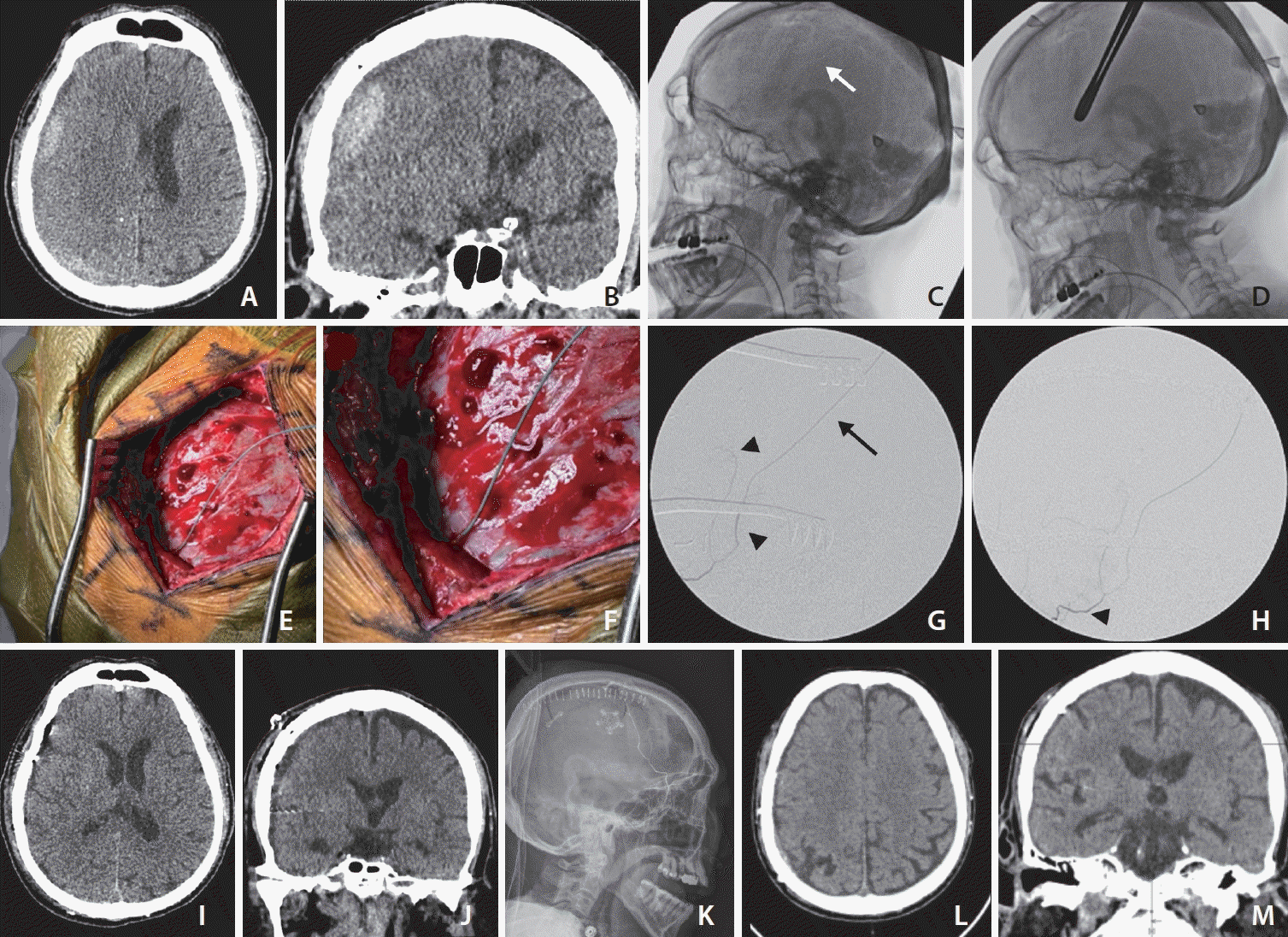 | Fig. 1.(A) Axial and (B) coronal views of computed tomography (CT) images of the head demonstrate a subacute subdural hematoma (SDH) that has partially liquefied 10 days after admission of the patient. (C) Intraoperativelateral skull X-ray. The white arrow points towards a prominent calvarial groove in which an middle meningeal artery (MMA) branch is expected to be found. (D) Lateral skull X-ray with sponge stick over the calvarial groove used to mark out the incision, note the pins of the radiolucent Mayfield head holder. (E, F) Direct catheterization of the frontal MMA branch on the dura at the craniotomy site. (G) Image of the anterior and posterior frontal MMA branches (black arrowheads). The black arrow points at the microcatheter. (H) Intraoperative digital subtraction angiography of the right middle meningeal artery. The black arrowhead points to the point from which the embolization was carried out. (I) Axial and (J) coronal views of CT images of the head demonstrate near resolution of the subacute on chronic SDH along the right cerebral convexity with improvement in midline shift on postoperative imaging. Onyx 18 cast can be seen in the main trunk of the right MMA. (K) Robust penetration of Onyx 18 embolic agent into right frontal branches of the MMA on the lateral skull X-ray. (L) Axial and (M) coronal views of a CT brain show near complete resolution of the right SDH 1.5 months after surgery. |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download