INTRODUCTION
METHODS
Study design
Study participants
Outcome assessment
Statistical analysis
RESULTS
Baseline characteristics
Table 1.
| Variable |
Group |
P value | |||
|---|---|---|---|---|---|
| Total (n=214) | Alo (n=75) | Pio (n=69) | Alo+Pio (n=70) | ||
| Age, yr | 58.9±9.2 | 58.9±8.7 | 58.12±9.9 | 59.7±9.0 | 0.6822a |
| Age <65 year | 144 (67.29) | 55 (73.33) | 45 (65.22) | 44 (62.86) | 0.3671 |
| Male sex | 122 (57.01) | 44 (58.67) | 41 (59.42) | 37 (52.86) | 0.6907 |
| Weight, kg | 67.4±11.2 | 67.8±11.7 | 69.3±11.3 | 65.2±10.2 | 0.1555a |
| BMI, kg/m2 | 25.4±3.3 | 25.5±3.3 | 25.7±3.3 | 25.1±3.4 | 0.4418a |
| Duration of T2DM, yr | 9.1±5.7 | 9.6 ± 5.6 | 8.1± 5.6 | 9.7± 5.8 | 0.1295a |
| Metformin dose, mg/day | 1,434.8±353.1 | 1,416.0±361.3 | 1,476.1±339.4 | 1,414.3±358.9 | 0.5226a |
| Hypertension | 128 (59.81) | 48 (64.00) | 40 (57.97) | 40 (57.14) | 0.6531 |
| Dyslipidemia | 169 (78.97) | 61 (81.33) | 51 (73.91) | 57 (81.43) | 0.4562 |
| Current smoker | 34 (15.89) | 10 (13.33) | 14 (20.29) | 10 (14.29) | 0.7615 |
| Alcohol | 96 (44.86) | 34 (45.33) | 26 (37.68) | 36 (51.43) | 0.2638 |
| HbA1c, % | 8.0±0.4 | 7.9±0.7 | 8.1±0.8 | 8.1±0.8 | 0.3238a |
| FBS, mg/dL | 160.1±38.4 | 155.8±36.3 | 159.3±41.8 | 165.5±36.9 | 0.0069a |
| Fasting C-peptide, ng/dL | 1.72±0.75 | 1.73±0.74 | 1.79±0.89 | 1.64±0.61 | 0.7908a |
| Glycoalbumin, % | 20.3±3.7 | 19.8±3.5 | 20.1±3.9 | 20.9±3.8 | 0.2220b |
| GA/HbA1c ratio | 2.5±0.4 | 2.5±0.3 | 2.5±0.4 | 2.6±0.4 | 0.3105b |
| Total cholesterol, mg/dL | 163.6±37.4 | 163.5±33.3 | 157.7±34.4 | 169.4±43.6 | 0.3751a |
| Triglyceride, mg/dL | 170.4±262.5 | 163.9±151.9 | 137.4±68.7 | 210.0±425.0 | 0.2791a |
| HDL-C, mg/dL | 51.6±11.7 | 50.3±11.0 | 52.9±13.5 | 51.8±10.6 | 0.4817a |
| LDL-C, mg/dL | 94.5±30.6 | 96.3±32.1 | 91.0±29.8 | 96.0±30.1 | 0.4464a |
Values are presented as mean±standard deviation or number (%).
Alo, alogliptin; Pio, pioglitazone; BMI, body mass index; T2DM, type 2 diabetes mellitus; HbA1c, glycosylated hemoglobin; FBS, fasting blood sugar; GA, glycoalbumin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Comparison of glycemic efficacy between alogliptin/pioglitazone combination therapy and alogliptin or pioglitazone mono add-on therapy
Fig. 1.
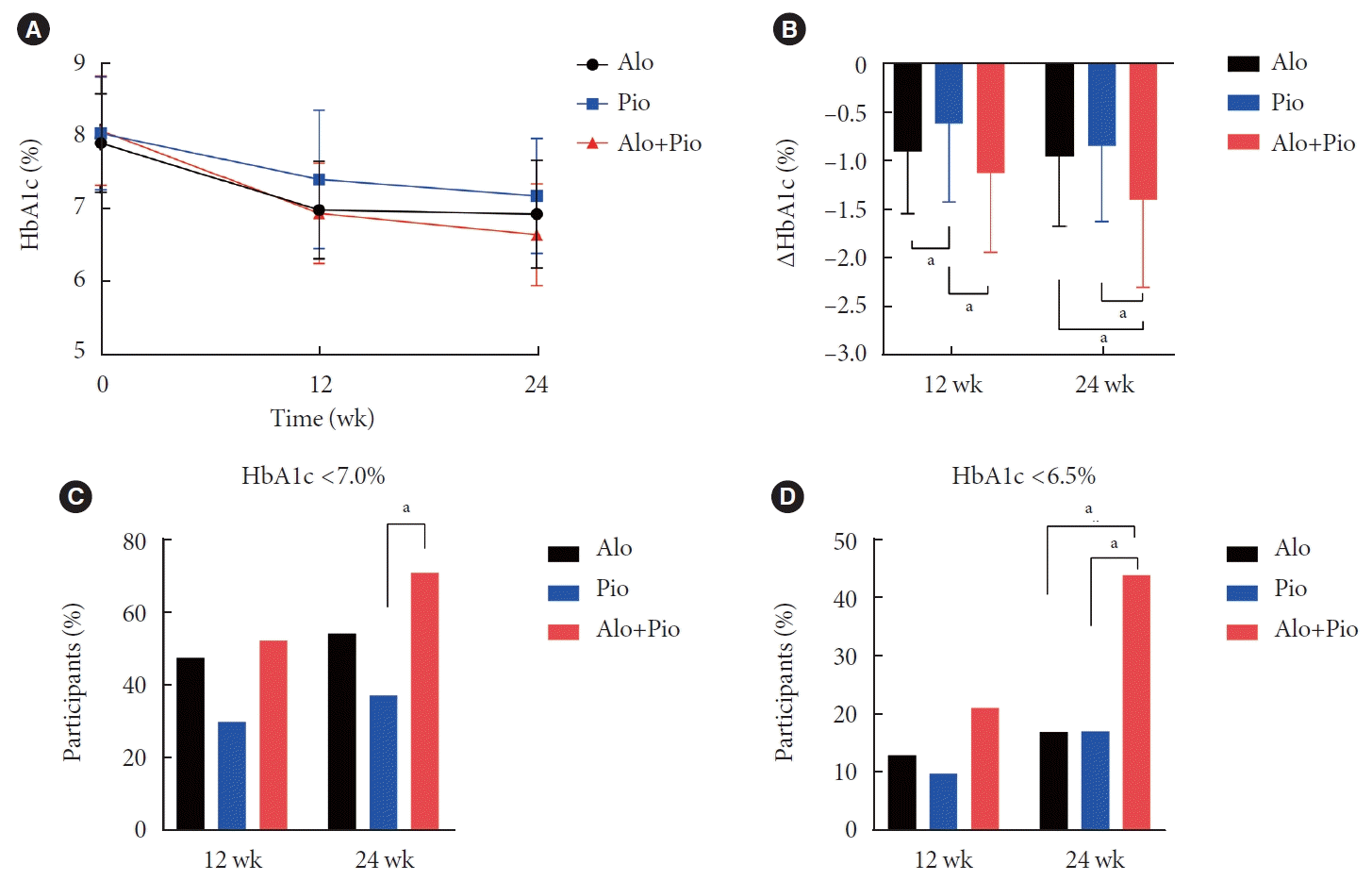
Comparison of metabolic parameters between alogliptin/pioglitazone combination therapy and alogliptin or pioglitazone mono add-on therapy
Table 2.
| Measurement |
Group |
P value | ||
|---|---|---|---|---|
| Alo (n=75) | Pio (n=69) | Alo+Pio (n=70) | ||
| Body weight, kg | ||||
| Baseline | 68.1±11.6 | 69.5±11.5 | 65.2±10.1 | |
| 12-week | 68.0±11.8 | 70.4±11.6 | 66.4±10.1 | |
| Change from baseline | –0.09±1.78 | 0.87±1.73 | 1.19±1.93 | <0.0001 |
| 24-week | 67.9±11.6 | 70.9±11.6 | 66.9±10.3 | |
| Change from baseline | –0.16±1.99 | 1.33±2.13 | 1.65±2.49 | <0.0001 |
| BMI, kg/m2 | ||||
| Baseline | 25.7±3.3 | 25.8±3.3 | 25.1±3.3 | |
| 12-week | 25.6±3.4 | 26.2±3.4 | 25.5±3.4 | |
| Change from baseline | –0.03±0.65 | 0.33±0.66 | 0.46±0.75 | <0.0001 |
| 24-week | 25.6±3.3 | 26.3±3.4 | 25.7±3.4 | |
| Change from baseline | –0.05±0.74 | 0.50±0.78 | 0.63±0.93 | <0.0001 |
| FBS, mg/dL | ||||
| Baseline | 155.8±36.3 | 159.3±41.8 | 165.5±36.9 | 0.0693a |
| 12-week | 134.7±24.3 | 136.4±32.8 | 123.4±24.1 | 0.0041a |
| Change from baseline | –24.0±2.85 | –23.41±2.98 | –38.27±2.96 | 0.0004a |
| 24-week | 133.3±27.3 | 134.4±33.9 | 122.6±24.5 | 0.0075a |
| Change from baseline | –25.57±3.10 | –25.43±3.22 | –39.00±3.21 | 0.0031a |
| Fasting insulin, μU/mL | ||||
| Baseline | 5.91±3.24 | 6.67±4.63 | 5.68±2.43 | 0.5672a |
| 12-week | 5.91±3.24 | 6.72±4.65 | 5.68±2.43 | 0.5225a |
| Change from baseline | –0.00±0.03 | 0.05±0.03 | –0.00±0.03 | 0.3560a |
| 24-week | 6.05±3.09 | 4.72±2.20 | 4.56±2.25 | 0.0063a |
| Change from baseline | 0.02±0.27 | –1.54±0.28 | –1.40±0.28 | <0.0001a |
| HOMA-β | ||||
| Baseline | 24.79±14.50 | 27.13±15.70 | 22.98±15.02 | 0.1784a |
| 24-week | 33.84±20.50 | 27.49±17.17 | 29.57±15.27 | 0.2415a |
| Change from baseline | 9.00±1.66 | 1.00±1.74 | 6.01±1.72 | 0.0042a |
| HOMA-IR | ||||
| Baseline | 2.37±1.73 | 2.77±2.86 | 2.32±1.17 | 0.4203a |
| 24-week | 2.05±1.28 | 1.56±0.78 | 1.45±0.91 | 0.0016a |
| Change from baseline | –0.41±0.11 | –0.97±0.12 | –1.00±0.12 | 0.0003a |
| Glycoalbumin, % | ||||
| Baseline | 19.8±3.5 | 20.1±3.9 | 20.9±3.8 | 0.2220b |
| 12-week | 16.6±2.7 | 18.4±4.3 | 16.5±3.2 | 0.0017a |
| Change from baseline | –3.34±0.32 | –1.74±0.33 | –4.07±0.33 | <0.0001a |
| 24-week | 17.0±3.1 | 18.2±3.7 | 16.8±3.3 | 0.0529a |
| Change from baseline | –3.01±0.31 | –2.02±0.32 | –3.82±0.32 | 0.0005a |
| GA/HbA1c ratio | ||||
| Baseline | 2.5±0.3 | 2.5±0.4 | 2.6±0.4 | 0.3105b |
| 12-week | 2.4±0.3 | 2.5±0.4 | 2.4±0.3 | 0.2201a |
| Change from baseline | –0.13±0.03 | –0.03±0.03 | –0.18±0.03 | 0.0016b |
| 24-week | 2.4±0.3 | 2.5±0.3 | 2.5±0.4 | 0.5703b |
| Change from baseline | –0.06±0.03 | 0.02±0.03 | –0.05±0.03 | 0.1610b |
Comparison of lipid profiles between alogliptin/ pioglitazone combination therapy and alogliptin or pioglitazone mono add-on therapy
Characteristics of patients who fail on alogliptin or pioglitazone combination therapy
Table 3.
| Variable |
Alogliptin |
Pioglitazone |
Alogliptin+Pioglitazone |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c <6.5% | HbA1c ≥6.5% | P value | HbA1c <6.5% | HbA1c ≥6.5% | P value | HbA1c <6.5% | HbA1c ≥6.5% | P value | |
| Number (%) | 13 (17.33) | 62 (82.67) | 12 (17.39) | 57 (82.61) | 31 (44.29) | 39 (55.71) | |||
| Age, yr | 57.69±7.98 | 59.13±8.87 | 0.5913 | 58.00±10.73 | 58.14±9.81 | 0.9684a | 61.16±9.12 | 58.62±8.85 | 0.2482a |
| Male sex | 10 (76.92) | 34 (54.84) | 0.1415 | 6 (50.00) | 35 (61.40) | 0.5274c | 15 (48.39) | 22 (56.41) | 0.5042b |
| Weight, kg | 68.95±10.04 | 67.49±12.02 | 0.5519a | 71.63±10.40 | 68.77±11.56 | 0.3224a | 66.06±10.78 | 64.48±9.81 | 0.5246 |
| BMI, kg/m2 | 26.05±3.71 | 25.42±3.27 | 0.5899a | 27.17±3.06 | 25.43±3.28 | 0.0595a | 25.76±3.97 | 24.49±2.75 | 0.1271a |
| Duration of T2DM, yr | 8.23±5.72 | 9.89±5.64 | 0.3918a | 6.50±5.39 | 8.40±5.68 | 0.2803a | 9.26±5.25 | 10.03±6.23 | 0.6740a |
| Metformin dose, mg/day | 1,323.08±363.21 | 1,435.48±360.83 | 0.3493a | 1,350.00±365.56 | 1,502.63±330.91 | 0.1979a | 1,400.00±347.37 | 1,425.64±371.85 | 0.7516a |
| Hypertension | 9 (69.23) | 39 (62.90) | 0.7594c | 9 (75.00) | 31 (54.39) | 0.1885b | 20 (64.52) | 20 (51.28) | 0.2664b |
| Dyslipidemia | 12 (92.31) | 49 (79.03) | 0.4402c | 10 (83.33) | 41 (71.93%) | 0.7184c | 25 (80.65) | 32 (82.05) | 0.8806b |
| Current smoker | 1 (7.69) | 9 (14.52) | 0.1107c | 2 (16.67) | 12 (21.05) | 0.0465c | 5 (16.13) | 5 (12.82) | 0.9198b |
| Alcohol | 46 (46.15) | 28 (45.16) | 0.9479b | 4 (33.33) | 22 (38.60) | 1.0000c | 14 (45.16) | 22 (56.41) | 0.3496b |
| HbA1c, % | 7.61±0.64 | 7.99±0.68 | 0.0222a | 7.48±0.34 | 8.19±0.79 | 0.0055a | 7.84±0.65 | 8.30±0.77 | 0.0066a |
| Glycoalbumin, % | 18.54±3.17 | 20.07±3.47 | 0.1458 | 17.44±3.70 | 20.69±3.73 | 0.0078 | 20.20±3.50 | 21.38±4.00 | 0.2008 |
| GA/HbA1c ratio | 2.43±0.26 | 2.51±0.34 | 0.4233 | 2.33±0.45 | 2.52±0.34 | 0.0986 | 2.57±0.35 | 2.57±0.36 | 0.9377 |
| FBS, mg/dL | 156.62±39.98 | 155.63±35.83 | 0.6899a | 138.33±15.67 | 163.65±44.22 | 0.0366a | 163.71±35.33 | 166.90±38.53 | 0.6362a |
| Fasting C-peptide, ng/dL | 1.99±0.98 | 1.67±0.68 | 0.2906a | 1.97±0.56 | 1.75±0.94 | 0.0469a | 1.77±0.58 | 1.54±0.63 | 0.0328a |
| HOMA-IR | 3.05±2.15 | 2.23±1.61 | 0.0678a | 2.59±0.87 | 2.80±3.13 | 0.1612a | 2.39±0.98 | 2.28±1.31 | 0.2820a |
| HOMA-β | 31.55±17.93 | 23.37±13.41 | 0.0678a | 37.28±12.95 | 24.99±15.48 | 0.0039a | 24.38±15.82 | 21.87±14.46 | 0.3149a |
| Total cholesterol, mg/dL | 150.08±31.43 | 166.26±33.25 | 0.1119 | 158.58±29.23 | 157.54±35.67 | 0.9251 | 168.87±52.98 | 169.82±35.06 | 0.4491a |
| Triglyceride, mg/dL | 230.46±328.87 | 149.90±74.67 | 0.5900a | 151.83±60.03 | 134.37±70.54 | 0.2319a | 274.26±618.92 | 158.95±139.76 | 0.1347a |
| HDL-C, mg/dL | 50.81±11.26 | 50.23±11.02 | 0.8337a | 53.86±16.28 | 52.72±12.97 | 0.6576a | 49.08±8.29 | 53.96±11.72 | 0.0537 |
| LDL-C, mg/dL | 77.46±31.11 | 100.26±31.05 | 0.0186 | 92.67±29.11 | 90.61±30.16 | 0.8300 | 92.48±31.27 | 98.82±29.19 | 0.3851 |
| Free fatty acid, μEq/L | 695.77±254.87 | 771.82±219.30 | 0.2726 | 721.00±222.05 | 751.30±257.40 | 0.7061 | 807.13±340.18 | 864.23±330.34 | 0.3979a |
Values are presented as number (%) or mean±standard deviation. The baseline characteristics of patients with treatment failure at 24 weeks.
HbA1c, glycosylated hemoglobin; BMI, body mass index; T2DM, type 2 diabetes mellitus; GA, glycoalbumin; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-β, homeostatic model assessment for β-function; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download