Abstract
Cardiovascular disease is a leading cause of global mortality, necessitating effective strategies for prevention and treatment. The cardiovascular disease continuum concept highlights the progression from risk factors such as hypertension and diabetes mellitus to advanced stages, including heart failure (HF) and death. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, initially developed to manage diabetes, have emerged as effective therapies across all stages of the cardiovascular disease continuum. Numerous cardiovascular outcome trials demonstrate that SGLT2 inhibitors significantly reduce major adverse cardiovascular events and hospitalizations for HF in patients with and without established atherosclerotic cardiovascular disease. Notably, SGLT2 inhibitors have shown remarkable benefits in reducing HF risk, even in patients without diabetes, including those with HF and preserved ejection fraction. Furthermore, recent studies in post–myocardial infarction patients suggest potential benefits in reducing hospitalizations for HF. Despite their widespread use, the precise mechanisms by which SGLT2 inhibitors confer cardiovascular protection remain unclear, suggesting the need for further investigation. In conclusion, SGLT2 inhibitors have revolutionized cardiovascular disease management, offering significant therapeutic potential across a broad spectrum of patients, and are expected to play an increasingly prominent role in both the prevention and treatment of cardiovascular disease.
Cardiovascular disease remains a leading cause of mortality worldwide, highlighting the urgent need for effective public health strategies targeting both prevention and treatment. The cornerstone of cardiovascular disease management lies in prevention, which focuses on identifying and mitigating risk factors [1]. Despite preventive efforts, many patients still develop vascular complications such as coronary artery disease, peripheral arterial disease, or cerebrovascular disease, which can lead to both reversible and irreversible organ dysfunction often manifesting as heart failure (HF), stroke, or ischemic limb loss. This progression from risk factors to vascular complications and subsequent end-organ damage highlights the complex, multifaceted nature of cardiovascular disease and underscores the need for comprehensive, long-term management strategies that address both prevention and treatment of established disease. A combination of pharmacological and nonpharmacological interventions is utilized to improve organ function, alleviate symptoms, enhance quality of life, and ultimately extend patients’ lifespans [2].
Diabetes mellitus is a major risk factor for cardiovascular disease [3–6]. Over the past four decades, numerous antidiabetic medications have been developed to prevent cardiovascular complications by achieving strict glycemic control [7]. Although these medications have successfully reduced blood glucose and hemoglobin A1c levels, and prevented acute diabetic complications such as hyperglycemic hyperosmolar state, they have had limited impact on reducing cardiovascular complications and mortality [8]. In addition, a 2007 meta-analysis showed that rosiglitazone increased the risk of cardiovascular death and HF, leading to its market withdrawal [9]. This finding raised concerns about the potential cardiovascular risks associated with diabetes medications, prompting the US Food and Drug Administration (FDA) to mandate evidence of cardiovascular safety for new antidiabetic drugs.
Sodium glucose cotransporter 2 (SGLT2) inhibitors were introduced in 2013 and function by lowering blood glucose levels through the inhibition of renal glucose reabsorption. To meet the FDA’s cardiovascular safety requirements, several large-scale cardiovascular outcome trials were conducted, including the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose) [10], CANVAS Program (Canagliflozin Cardiovascular Assessment Study Program) [11], DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58) [12], and VERTIS CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes) [13]. These trials consistently showed that SGLT2 inhibitors not only met safety standards compared to placebo but also significantly reduced the incidence of major adverse cardiovascular events (MACE) as well as hospitalizations for HF (HHF). These unexpected findings have marked a turning point in the role of SGLT2 inhibitors in cardiovascular care.
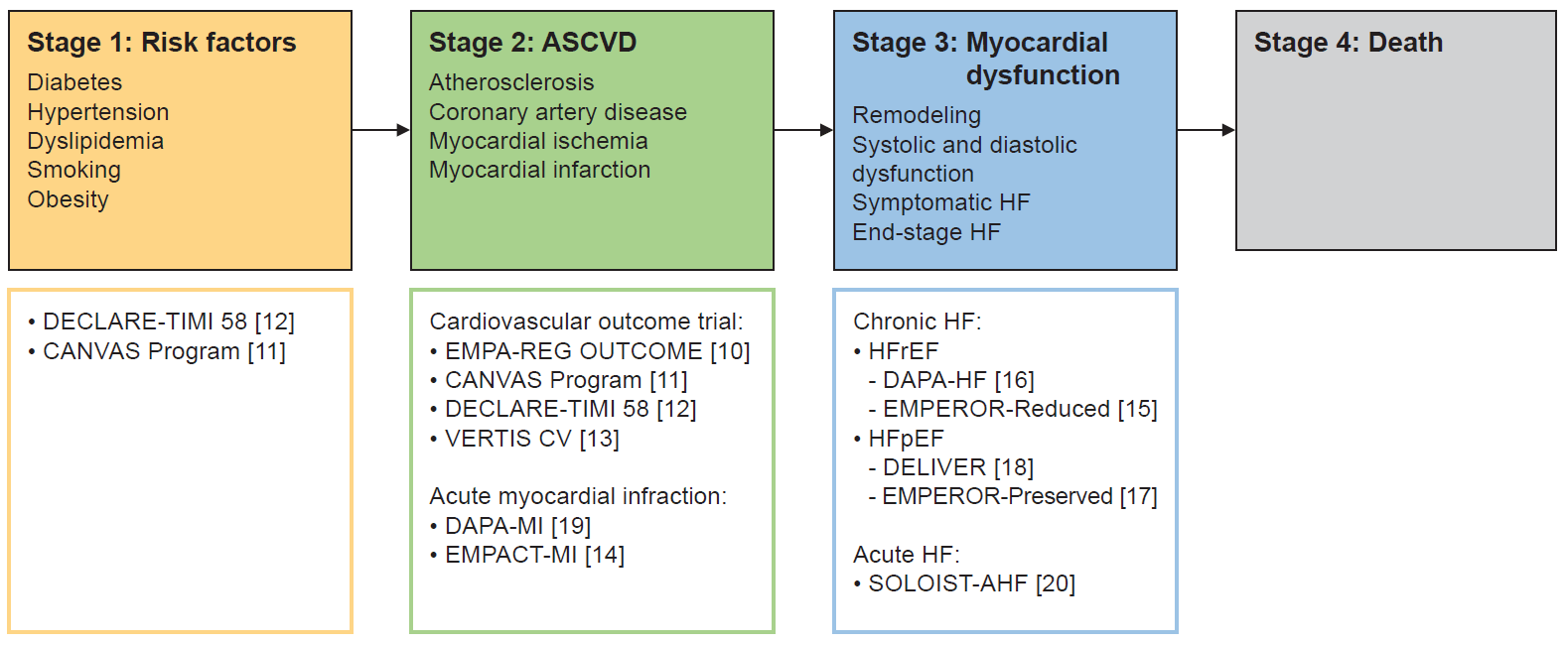
The cardiovascular disease continuum is a conceptual framework that views the development and progression of cardiovascular disease as a continuous series of stages, beginning with the emergence of early risk factors and advancing to irreversible cardiac damage and, ultimately, death. This concept emphasizes how early risk factors such as hypertension, diabetes mellitus, and dyslipidemia can lead to endothelial dysfunction and atherosclerosis over time, potentially resulting in serious cardiovascular conditions like coronary artery disease, myocardial infarction (MI), and HF. The primary goal of this framework is to intervene at each stage, halting disease progression and improving patient outcomes through targeted preventive and therapeutic strategies. Understanding this continuum is essential for developing preventive measures, early diagnostic techniques, and treatment strategies, enabling a comprehensive approach to managing cardiovascular disease. The continuum is divided into four primary stages: (1) risk factors; (2) atherosclerotic cardiovascular disease (ASCVD); (3) myocardial dysfunction; and (4) death (Fig. 1) [10–20]. This review provides an in-depth analysis of the effects of SGLT2 inhibitors across each of these stages.
To date, no cardiovascular outcome trials have specifically targeted patients in the early stages of the cardiovascular disease continuum—those presenting only with risk factors for cardiovascular disease. However, the DECLARE-TIMI 58 study [12] and the CANVAS Program [11] have explored the efficacy and safety of SGLT2 inhibitors in high-risk patients without established ASCVD. The DECLARE-TIMI 58 study evaluated the cardiovascular safety of dapagliflozin versus placebo in 17,160 patients with type 2 diabetes [12]. This cohort included 6,974 patients undergoing secondary prevention with ASCVD and 10,186 patients undergoing primary prevention without ASCVD. In a subgroup analysis focusing solely on primary prevention patients, the incidence of MACE showed no significant difference between the dapagliflozin and placebo groups (hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.86–1.20). However, the dapagliflozin group exhibited a 16% reduction in the composite endpoint of cardiovascular death and HHF compared to the placebo group (HR, 0.84; 95% CI, 0.67–1.04). Similarly, the CANVAS Program study assessed the safety of canagliflozin in a cohort of 10,142 patients with type 2 diabetes, including 6,656 patients with cardiovascular disease and 3,486 without [11]. A subgroup analysis of the 3,486 patients without cardiovascular disease revealed comparable MACE rates between the canagliflozin and placebo groups (HR, 0.98; 95% CI, 0.74–1.30). However, the canagliflozin group demonstrated a 14% reduction in the incidence of cardiovascular death and HHF compared to the placebo group (HR, 0.83; 95% CI, 0.58–1.19). A meta-analysis of patients without ASCVD from both CANVAS Program [11] and DECLARE-TIMI 58 [12] showed that SGLT2 inhibitors were associated with a 16% reduction in the risk of cardiovascular death and HHF (HR, 0.84; 95% CI, 0.69–1.01) [21]. These results indicate that while SGLT2 inhibitors have a neutral effect on MACE in patients with type 2 diabetes without ASCVD, there is a trend toward a reduced risk of cardiovascular death and HHF. This suggests that SGLT2 inhibitors may offer benefits even in the early stages of the cardiovascular disease continuum.
Several landmark studies have demonstrated the cardiovascular benefits of SGLT2 inhibitors in patients with type 2 diabetes and established ASCVD. The EMPA-REG OUTCOME study, which included 7,020 patients with type 2 diabetes and established cardiovascular disease (76% with coronary artery disease and 23% with a history of stroke), evaluated the cardiovascular safety of empagliflozin over a 48-month follow-up period [10]. The results revealed that empagliflozin significantly reduced the composite of cardiovascular death and 3-point MACE by 14% compared to placebo. Similarly, the DECLARE-TIMI 58 study, involving 6,974 patients with ASCVD, demonstrated that dapagliflozin reduced the incidence of 3-point MACE by 10% compared to placebo (HR, 0.90; 95% CI, 0.79–1.02). Moreover, it decreased the risk of cardiovascular death and HHF by 17% (HR, 0.83; 95% CI, 0.71–0.98) [12]. A comprehensive meta-analysis of 20,650 ASCVD patients from EMPA-REG OUTCOME [10], CANVAS Program [11], and DECLARE-TIMI 58 [12] further solidified these findings [21]. It demonstrated that SGLT2 inhibitors reduced the incidence of MACE by 14% (HR, 0.86; 95% CI, 0.80–0.93) and decreased cardiovascular death and HHF by 24% (HR, 0.76; 95% CI, 0.69–0.84).
These consistent results across multiple large-scale trials provide robust evidence that SGLT2 inhibitors are effective in both preventing and treating cardiovascular disease in diabetic patients with ASCVD. The significant reductions in MACE and HHF underscore the potential of SGLT2 inhibitors as a valuable therapeutic option in managing cardiovascular risk in this high-risk population.
The recent DAPA-MI (Dapagliflozin Myocardial Infarction) study, a European registry-based randomized clinical trial, explored the effectiveness of dapagliflozin in 4,017 patients who did not have diabetes or chronic HF but had suffered an acute MI within the past 10 days and exhibited reduced left ventricular systolic function [19]. The primary outcome was assessed using a win ratio of composite variables, including death, HHF, nonfatal MI, atrial fibrillation/arrhythmia, the onset of type 2 diabetes, New York Heart Association classification of HF symptoms, and a 5% or greater weight loss at the final visit. The study showed that patients in the dapagliflozin group were 34% more likely to achieve better cardiometabolic outcomes compared to those in the placebo group. However, dapagliflozin did not significantly affect traditional clinical endpoints, such as cardiovascular death or HHF (HR, 0.95; 95% CI, 0.64–1.40).
The EMPACT-MI (Effect of Empagliflozin on Hospitalization for Heart Failure and Mortality in Patients with Acute Myocardial Infarction) study was a large, international, randomized, double-blind, placebo-controlled trial that evaluated the effects of empagliflozin in 6,522 patients who had recently experienced an acute MI within 14 days and were at high risk of developing HF [14]. The primary outcome—a composite of all-cause mortality and first HHF—showed no significant difference between the empagliflozin and placebo groups (HR, 0.90; 95% CI, 0.76–1.06). However, secondary analyses suggested that empagliflozin may have reduced both first and total HHF, indicating potential benefits for HF outcomes in this high-risk post-MI population.
Taken together, SGLT2 inhibitors appear to reduce the risk of HF in post-MI patients.
Cardiovascular outcome trials for SGLT2 inhibitors, including EMPA-REG OUTCOME [10], the CANVAS Program [11], DECLARE-TIMI 58 [12] and VERTIS CV [13], have consistently demonstrated a reduction in HHF. As a result, dedicated studies have been conducted in HF patients to confirm the efficacy and safety of SGLT2 inhibitors for these patients.
HF is categorized based on left ventricular ejection fraction (LVEF) into three types: HF with reduced ejection fraction (HFrEF), HF with mildly reduced ejection fraction, and HF with preserved ejection fraction (HFpEF) [22,23]. Medications such as angiotensin-converting enzyme inhibitors [24], angiotensin receptor neprilysin inhibitors [25], β-blockers [26], and mineralocorticoid receptor antagonists [27] have been shown to effectively reduce the risk of cardiovascular death in patients with HFrEF and are recommended as first-line treatments [28]. However, these drugs have shown limited efficacy in HFpEF patients, and thus, they are not recommended as first-line therapies for managing this condition [22,29].
The EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction) [15] and the DAPA-HF (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure) [16] studies have demonstrated the effectiveness of SGLT2 inhibitors in improving outcomes for HF patients. The EMPEROR-Reduced study compared empagliflozin 10 mg with placebo in 3,730 HFrEF patients, including those with and without type 2 diabetes. It found that the risk of cardiovascular death or HHF was 19.4% in the empagliflozin group compared to 24.7% in the placebo group, representing a 25% risk reduction (HR, 0.75; 95% CI, 0.65–0.86). This benefit was consistent regardless of the presence of type 2 diabetes at enrollment (diabetes group: HR, 0.72 [95% CI, 0.60–0.87]; nondiabetes group: HR, 0.78 [95% CI, 0.64–0.97]). The DAPA-HF study assessed the efficacy of dapagliflozin in 4,744 HFrEF patients, approximately 45% of whom were diabetic [16]. This study showed that dapagliflozin reduced the primary endpoint—a composite of cardiovascular death or HHF—by a similar degree (HR, 0.74; 95% CI, 0.65–0.85) regardless of diabetes status.
Studies on HFpEF patients include the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) [17] and the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) [18]. The EMPEROR-Preserved study compared empagliflozin 10 mg with placebo in 5,988 HFpEF patients with an LVEF of 40% or higher [17]. It demonstrated a 21% reduction in the primary endpoint of cardiovascular death or HHF (HR, 0.79; 95% CI, 0.69–0.90). The DELIVER study assessed the efficacy of dapagliflozin in 6,263 chronic HF patients with an LVEF of 40% or higher [18]. Dapagliflozin was found to reduce the risk of the composite endpoint of cardiovascular death or worsening HF by 18% compared to placebo (HR, 0.82; 95% CI, 0.73–0.92).
A meta-analysis including patients from both EMPEROR-Preserved [17] and DELIVER [18] demonstrated a 20% reduction in cardiovascular death and HHF (HR, 0.80; 95% CI, 0.73–0.87). Based on these results, HF management guidelines from Korea, the United States, and Europe now recommend SGLT2 inhibitors as first-line therapies [2,22,30]. Importantly, the benefits of SGLT2 inhibitors were observed consistently, irrespective of the patients' diabetes status.
Clinical studies have consistently demonstrated the cardiovascular benefits of SGLT2 inhibitors. However, the mechanisms underlying these effects are not yet fully understood. Notably, even patients without diabetes have experienced cardioprotective benefits, indicating that mechanisms other than glucose-lowering may be significant. Therefore, it is essential to clarify the mechanisms of action of SGLT2 inhibitors, as this could significantly enhance hypothesis-driven validation of drug efficacy and the development of more effective agents.
Although SGLT2 inhibitors were initially developed to treat type 2 diabetes, they have proven beneficial at all stages of cardiovascular disease. They are particularly effective in preventing the onset and progression of HF. Consequently, SGLT2 inhibitors have become essential first-line treatments for HF patients, regardless of their diabetes status. They are also the only recognized effective therapy for patients with HFpEF, a condition previously lacking effective treatment. These cardiovascular protective effects have also been observed in patients without diabetes, suggesting that SGLT2 inhibitors have a broader application than existing cardiovascular medications. Therefore, they are expected to play a significant role in the prevention and treatment of cardiovascular disease. However, the exact mechanisms of action of these drugs are not yet fully understood. Therefore, further research is necessary, which may potentially reveal new uses for these drugs.
Notes
REFERENCES
1. Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006; 114:2850–70.

2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022; 79:e263–421.
3. Park JJ. Epidemiology, pathophysiology, diagnosis and treatment of heart failure in diabetes. Diabetes Metab J. 2021; 45:146–57.

4. Lee KS, Noh J, Park SM, Choi KM, Kang SM, Won KC, et al. Evaluation and management of patients with diabetes and heart failure: a Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement. Int J Heart Fail. 2023; 5:1–20.

5. Lee HY. Heart failure and diabetes mellitus: dangerous liaisons. Int J Heart Fail. 2022; 4:163–74.

6. Lee CJ, Lee H, Yoon M, Chun KH, Kong MG, Jung MH, et al. Heart failure statistics 2024 update: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2024; 6:56–69.

7. Choi JH, Lee KA, Moon JH, Chon S, Kim DJ, Kim HJ, et al. 2023 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2023; 47:575–94.

8. Control Group, Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009; 52:2288–98.

9. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–71.

10. Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2016; 374:1094.

11. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–57.

12. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–57.

13. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020; 383:1425–35.

14. Butler J, Jones WS, Udell JA, Anker SD, Petrie MC, Harrington J, et al. Empagliflozin after Acute Myocardial Infarction. N Engl J Med. 2024; 390:1455–66.
15. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–24.
16. McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008.
17. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385:1451–61.
18. Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 2022; 10:184–97.
19. James S, Erlinge D, Storey RF, McGuire DK, de Belder M, Eriksson N, et al. Dapagliflozin in myocardial infarction without diabetes or heart failure. NEJM Evid. 2024; 3:EVIDoa2300286.

20. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021; 384:117–128.

21. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–9.

22. Youn JC, Kim D, Cho JY, Cho DH, Park SM, Jung MH, et al. Korean Society of Heart Failure guidelines for the management of heart failure: treatment. Int J Heart Fail. 2023; 5:66–81.

23. Cho JY, Cho DH, Youn JC, Kim D, Park SM, Jung MH, et al. Korean Society of Heart Failure guidelines for the management of heart failure: definition and diagnosis. Int J Heart Fail. 2023; 5:51–65.

24. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991; 325:293–302.

25. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371:993–1004.

26. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996; 334:1349–55.

27. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999; 341:709–17.

28. Yoo BS. President’s message: 4 pillars of Korean Society of Heart Failure. Int J Heart Fail. 2024; 6:117–8.

29. Park SM, Lee SY, Jung MH, Youn JC, Kim D, Cho JY, et al. Korean Society of Heart Failure guidelines for the management of heart failure: management of the underlying etiologies and comorbidities of heart failure. Int J Heart Fail. 2023; 5:127–45.

30. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2023 Focused update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023; 44:3627–39.
Fig. 1.
The cardiovascular disease continuum, illustrating the progression from early risk factors to advanced stages of cardiovascular disease. Key sodium-glucose cotransporter 2 (SGLT2) inhibitor clinical trials are mapped to each stage, demonstrating their effects across the continuum. ASCVD, atherosclerotic cardiovascular disease; HF, heart failure; DECLARE-TIMI 58, Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58; CANVAS Program, Canagliflozin Cardiovascular Assessment Study Program; EMPA-REG OUTCOME, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose; VERTIS CV, Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes; DAPA-MI, Dapagliflozin in Myocardial Infarction; EMPACT-MI, Effect of Empagliflozin on Hospitalization for Heart Failure and Mortality in Patients with Acute Myocardial Infarction; HFrEF, heart failure with reduced ejection fraction; DAPA-HF, Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction; HFpEF, heart failure with preserved ejection fraction; DELIVER, Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; SOLOIST-AHF, Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download