1. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022; 54:330–44.

2. Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004; 101:281–8.

4. Rao S, Guren MG, Khan K, Brown G, Renehan AG, Steigen SE, et al. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021; 32:1087–100.

5. Theophanous S, Samuel R, Lilley J, Henry A, Sebag-Montefiore D, Gilbert A, et al. Prognostic factors for patients with anal cancer treated with conformal radiotherapy-a systematic review. BMC Cancer. 2022; 22:607.

6. Rodel F, Steinhauser K, Kreis NN, Friemel A, Martin D, Wieland U, et al. Prognostic impact of RITA expression in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Radiother Oncol. 2018; 126:214–21.

7. Balermpas P, Martin D, Wieland U, Rave-Frank M, Strebhardt K, Rodel C, et al. Human papilloma virus load and PD-1/PD-L1, CD8(+) and FOXP3 in anal cancer patients treated with chemoradiotherapy: Rationale for immunotherapy. Oncoimmunology. 2017; 6:e1288331.
8. Schernberg A, Escande A, Rivin Del Campo E, Ducreux M, Nguyen F, Goere D, et al. Leukocytosis and neutrophilia predicts outcome in anal cancer. Radiother Oncol. 2017; 122:137–45.

9. Schernberg A, Huguet F, Moureau-Zabotto L, Chargari C, Rivin Del Campo E, Schlienger M, et al. External validation of leukocytosis and neutrophilia as a prognostic marker in anal carcinoma treated with definitive chemoradiation. Radiother Oncol. 2017; 124:110–7.

10. Kim E, Kim TH, Jung W, Kim K, Chang AR, Park HJ, et al. Prognostic impact of neutrophilia and lymphopenia on survival in anal cancer treated with definitive concurrent chemoradiotherapy: a retrospective multicenter study. Int J Clin Oncol. 2022; 27:553–62.

11. Shakir R, Adams R, Cooper R, Downing A, Geh I, Gilbert D, et al. Patterns and predictors of relapse following radical chemoradiation therapy delivered using intensity modulated radiation therapy with a simultaneous integrated boost in anal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2020; 106:329–39.

12. Janczewski LM, Faski J, Nelson H, Gollub MJ, Eng C, Brierley JD, et al. Survival outcomes used to generate version 9 American Joint Committee on Cancer staging system for anal cancer. CA Cancer J Clin. 2023; 73:516–23.

13. Tiwari R, Narayanan GS, Reddy VP, Vishwanathan B, Narayanan S, Venugopal R. Impact of nodal boost irradiation and MR-based brachytherapy on oncologic outcomes in node-positive cervical cancer. Gynecol Oncol. 2021; 163:110–6.

14. Grigsby PW, Singh AK, Siegel BA, Dehdashti F, Rader J, Zoberi I. Lymph node control in cervical cancer. Int J Radiat Oncol Biol Phys. 2004; 59:706–12.

15. O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016; 17:440–51.

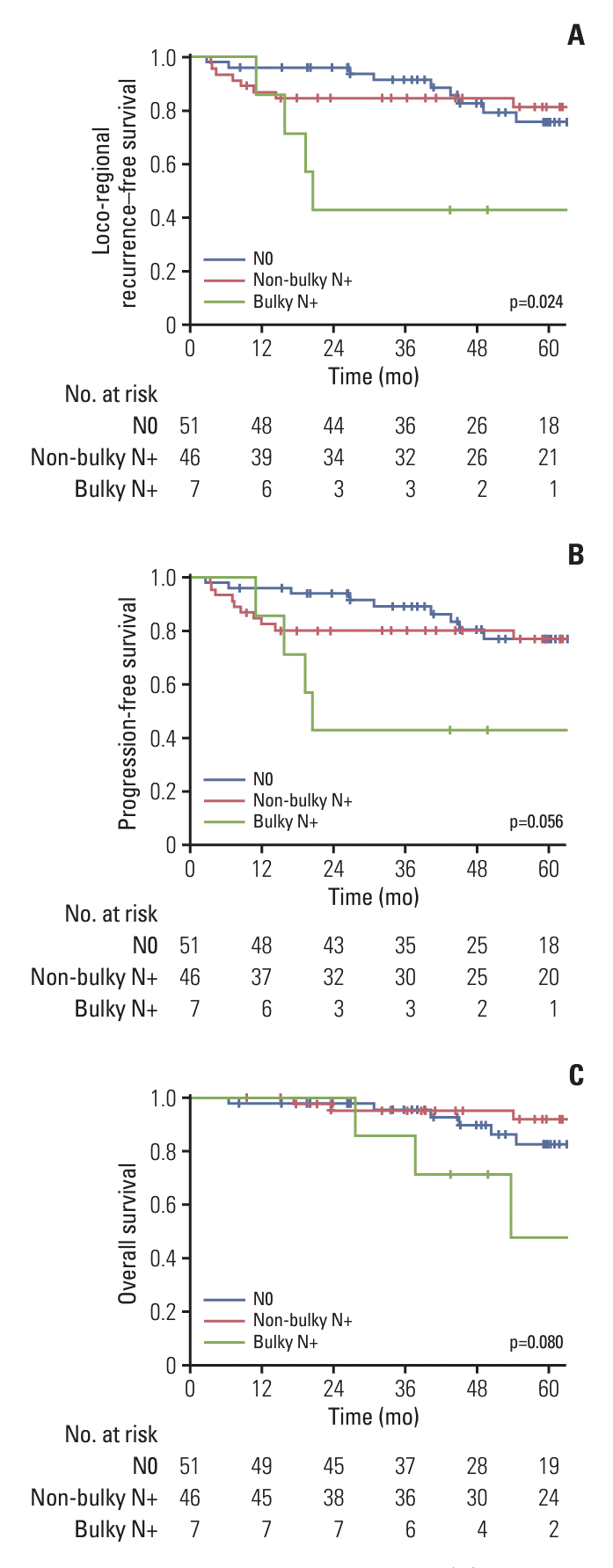
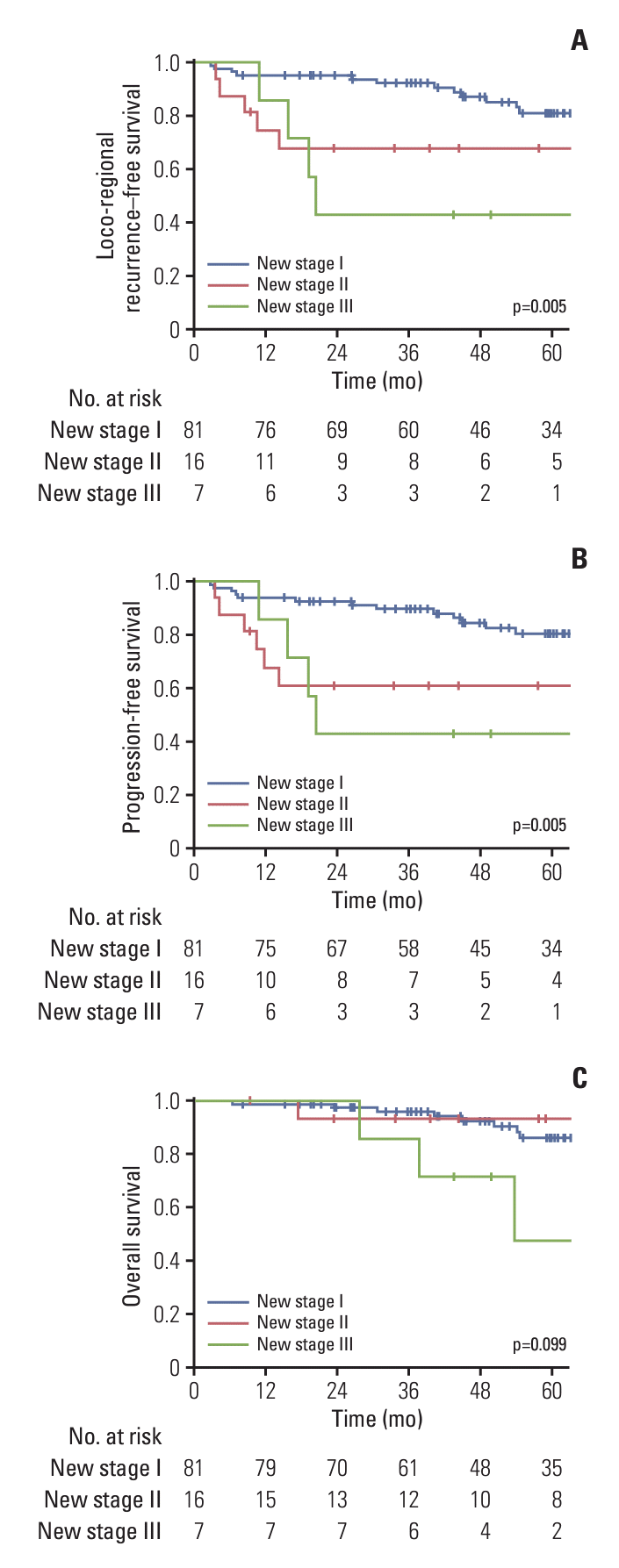
16. Chie EK, Chun SJ, Kim E, Jang WI, Kim MS, Kang HC, et al. Bulky nodal disease as distinctive prognosticator in anal cancer management. J Clin Oncol. 2024; 42(3 Suppl):1.

17. Peiffert D, Tournier-Rangeard L, Gerard JP, Lemanski C, Francois E, Giovannini M, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012; 30:1941–8.

18. Glynne-Jones R, Meadows HM, Lopes A, Muirhead R, SebagMontefiore D, Adams R, et al. Impact of compliance to chemoradiation on long-term outcomes in squamous cell carcinoma of the anus: results of a post hoc analysis from the randomised phase III ACT II trial. Ann Oncol. 2020; 31:1376–85.

19. Gilbert A, McParland L, Webster J, Bell S, Copeland J, Adams RA, et al. Pre-specified pilot analysis of a randomised pilot/phase II/III trial comparing standard dose vs dose-escalated concurrent chemoradiotherapy (CRT) in anal cancer (PLATOACT5). Ann Oncol. 2019; 30(Suppl 5):V203–4.

20. Gilbert DC, Wakeham K, Langley RE, Vale CL. Increased risk of second cancers at sites associated with HPV after a prior HPV-associated malignancy, a systematic review and meta-analysis. Br J Cancer. 2019; 120:256–68.

21. Sun G, Dong X, Tang X, Qu H, Zhang H, Zhao E. The prognostic value of HPV combined p16 status in patients with anal squamous cell carcinoma: a meta-analysis. Oncotarget. 2018; 9:8081–8.

22. Spector ME, Gallagher KK, Bellile E, Chinn SB, Ibrahim M, Byrd S, et al. Patterns of nodal metastasis and prognosis in human papillomavirus-positive oropharyngeal squamous cell carcinoma. Head Neck. 2014; 36:1233–40.

23. Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, et al. Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol. 2017; 35:3601–9.

24. Olthof EP, Wenzel H, van der Velden J, Spijkerboer AM, Bekkers R, Beltman JJ, et al. Treatment of bulky lymph nodes in locally advanced cervical cancer: boosting versus debulking. Int J Gynecol Cancer. 2022; 32:861–8.

25. Li P, Fang Q, Yang Y, Chen D, Du W, Liu F, et al. Survival significance of number of positive lymph nodes in oral squamous cell carcinoma stratified by p16. Front Oncol. 2021; 11:545433.

26. Bauwens L, Baltres A, Fiani DJ, Zrounba P, Buiret G, Fleury B, et al. Prevalence and distribution of cervical lymph node metastases in HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma. Radiother Oncol. 2021; 157:122–9.

27. Porceddu SV, Milne R, Brown E, Bernard A, Rahbari R, Cartmill B, et al. Validation of the ICON-S staging for HPV-associated oropharyngeal carcinoma using a pre-defined treatment policy. Oral Oncol. 2017; 66:81–6.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download