1. Scoccianti S, Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol. 2012; 102:168–79.

2. Kim T, Song C, Han JH, Kim IA, Kim YJ, Kim SH, et al. Epidemiology of intracranial metastases in Korea: a national cohort investigation. Cancer Res Treat. 2018; 50:164–74.

3. Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022; 66:15–23.

4. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011; 22:1–6.

5. Hatiboglu MA, Akdur K, Sawaya R. Neurosurgical management of patients with brain metastasis. Neurosurg Rev. 2020; 43:483–95.

6. Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY. Dural metastases. J Neurooncol. 2005; 75:57–61.

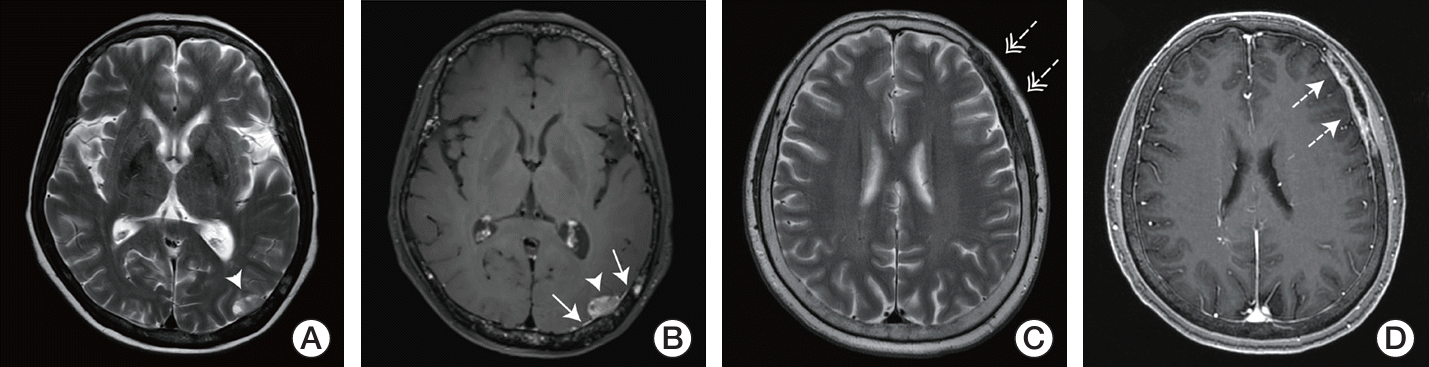
7. Agarwal B, Das P, Nasim M. Dural metastatic cancer from primary breast carcinoma. Int J Neurosci. 2010; 120:442–6.

8. Seki S, Kamide T, Tamase A, Mori K, Yanagimoto K, Nomura M. Intraparenchymal hemorrhage from dural metastasis of breast cancer mimicking meningioma. Neuroradiol J. 2016; 29:179–82.

9. Richiello A, Sparano L, Del Basso De Caro ML, Russo G. Dural metastasis mimicking falx meningioma: case report. J Neurosurg Sci. 2003; 47:167–71.
10. Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer. 2009; 115:1947–53.

11. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001; 27:165–76.

12. Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Beyond an updated graded prognostic assessment (Breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020; 107:334–43.

13. Riecke K, Muller V, Neunhoffer T, Park-Simon TW, Weide R, Polasik A, et al. Long-term survival of breast cancer patients with brain metastases: subanalysis of the BMBC registry. ESMO Open. 2023; 8:101213.

14. Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018; 25:1783–5.

15. Pareek A, Singh OP, Yogi V, Ghori HU, Tiwari V, Redhu P. Bone metastases incidence and its correlation with hormonal and human epidermal growth factor receptor 2 neu receptors in breast cancer. J Cancer Res Ther. 2019; 15:971–5.

16. Jiang X, Chen G, Sun L, Liu C, Zhang Y, Liu M, et al. Characteristics and survival in bone metastatic breast cancer patients with different hormone receptor status: a population-based cohort study. Front Oncol. 2022; 12:977226.

17. Aurilio G, Monfardini L, Rizzo S, Sciandivasci A, Preda L, Bagnardi V, et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. 2013; 52:1649–56.

18. Hilton JF, Amir E, Hopkins S, Nabavi M, DiPrimio G, Sheikh A, et al. Acquisition of metastatic tissue from patients with bone metastases from breast cancer. Breast Cancer Res Treat. 2011; 129:761–5.

19. Hofbauer LC, Rachner TD, Coleman RE, Jakob F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014; 2:500–12.

20. Zimmer AS, Van Swearingen AE, Anders CK. HER2-positive breast cancer brain metastasis: a new and exciting landscape. Cancer Rep (Hoboken). 2022; 5:e1274.

21. Kyeong S, Cha YJ, Ahn SG, Suh SH, Son EJ, Ahn SJ. Subtypes of breast cancer show different spatial distributions of brain metastases. PLoS One. 2017; 12:e0188542.

22. Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007; 67:4190–8.

23. Sirkisoon SR, Carpenter RL, Rimkus T, Miller L, Metheny-Barlow L, Lo HW. EGFR and HER2 signaling in breast cancer brain metastasis. Front Biosci (Elite Ed). 2016; 8:245–63.

24. Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N. Wholebrain radiation therapy for breast cancer patients with dural metastasis without concomitant brain metastasis and leptomeningeal metastasis. Anticancer Res. 2018; 38:6405–11.

25. Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010; 136:1729–35.

26. Boogerd W, van den Bent MJ, Koehler PJ, Heimans JJ, van der Sande JJ, Aaronson NK, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004; 40:2726–33.

27. van der Ree TC, Dippel DW, Avezaat CJ, Sillevis Smitt PA, Vecht CJ, van den Bent MJ. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999; 66:225–7.

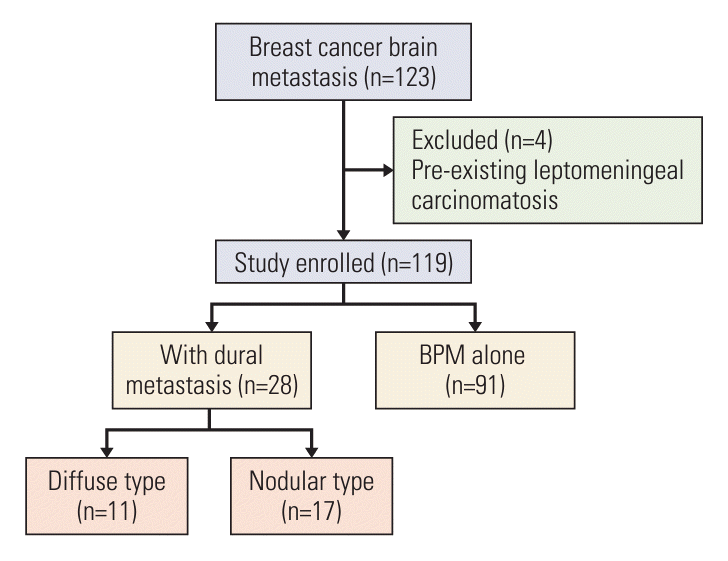
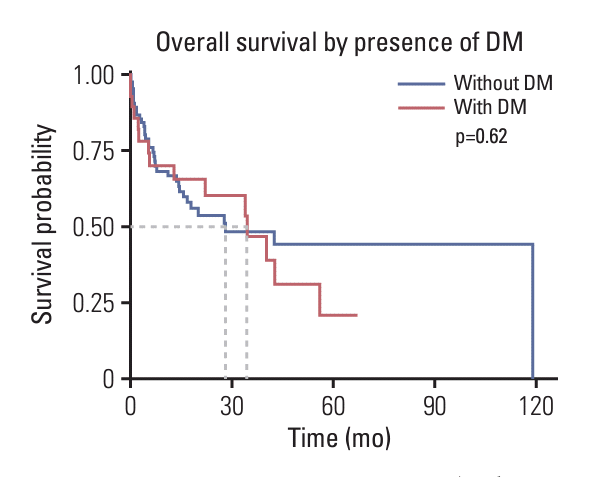
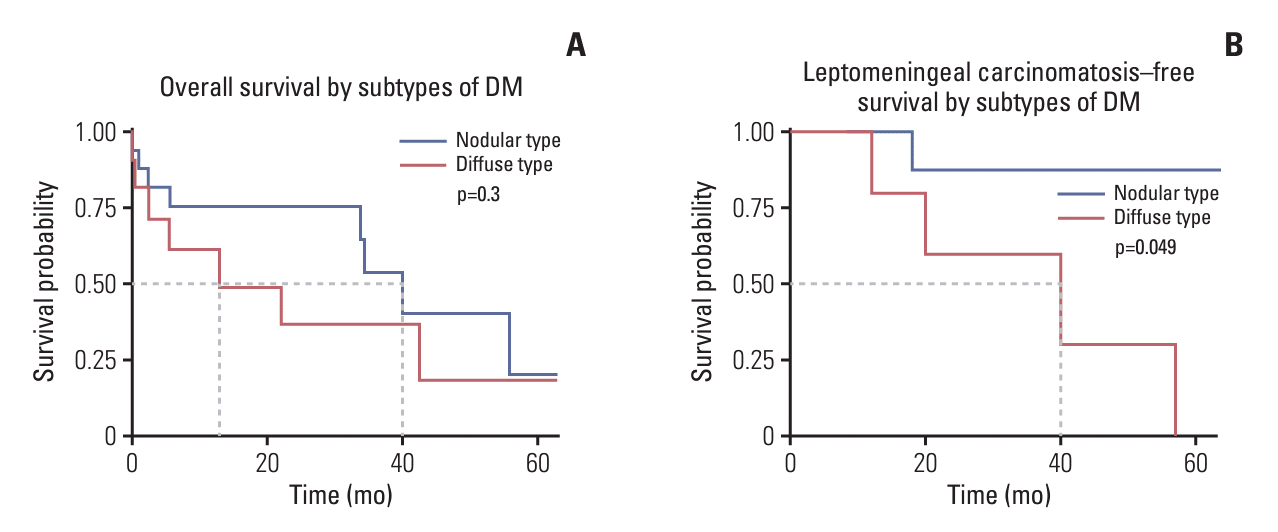
28. Jung JM, Kim S, Joo J, Shin KH, Gwak HS, Lee SH. Incidence and risk factors for leptomeningeal carcinomatosis in breast cancer patients with parenchymal brain metastases. J Korean Neurosurg Soc. 2012; 52:193–9.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download