Introduction
Materials and Methods
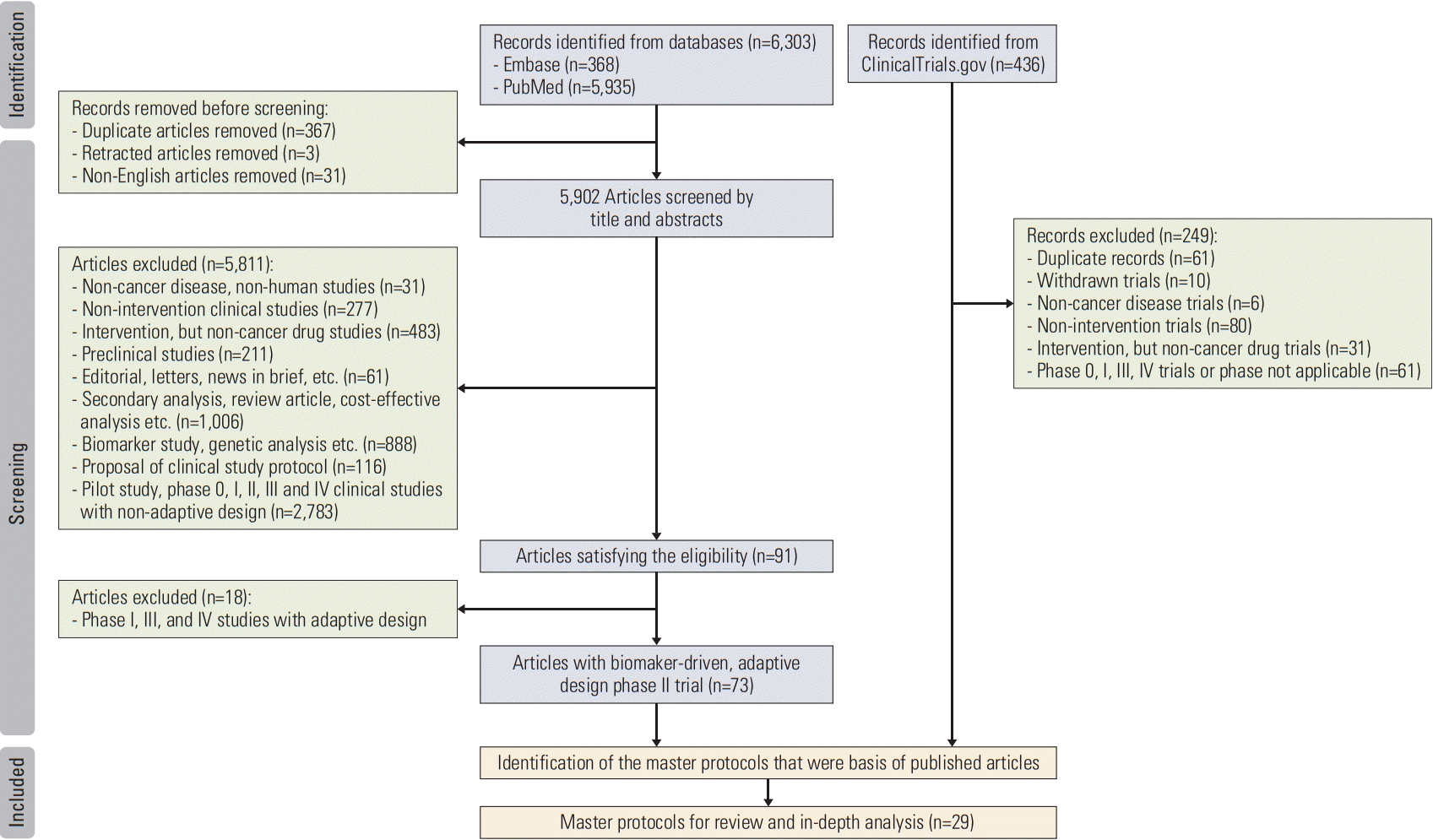
1. Literature search and study selection
2. Eligibility criteria
3. Categorization of POCTs
Results
1. Infrastructure
Table 1.
ANR, Agence Nationale de le Recherche; ASCO, American Society of Clinical Oncology; CRCTU, Cancer Research UK Clinical Trials Unit; CRUK, Cancer Research UK; DRUP, Drug Rediscovery Protocol; EORTC, European Organisation for Research and Treatment of Cancer; INCa, Institut National du Cancer; MATCH, Molecular Analysis for Therapy Choice; MRC CTU, Medical Research Council Clinical Trials Unit; MSKCC, Memorial Sloan Kettering Cancer Center; NCI, National Cancer Institute; NCTN, National Clinical Trials Network; NIH, National Institute of Health; NIHR, National Institute for Health and Care Research; NRF, National Research Foundation of Korea; SiRIC, Site de Recherche Intégré contre le Cancer; SUKSES, Small cell lung cancer Umbrella Korea StudiES; SWOG, Southwest Cancer Chemotherapy Study Group; TAPUR, Targeted Agent and Profiling Utilization Registry.
Table 2.
2. Biomarker screening
Table 3.
| Study title | Year started | Target disease(s) | Screening method(s) | Specimen type | Screening lab | Results reviewed by | |
|---|---|---|---|---|---|---|---|
| Basket trial | |||||||
| IIT | Ado-Trastuzumab Emtansine | 2016 | HER2-mutant or -amplified, advanced solid tumors | NGS | Tissue | Local | N/A |
| Basket Trial | |||||||
| ADVL1522 | 2015 | Recurrent or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, MPNST, or synovial sarcoma in pts between 12 months-30 years old | IHC | Tissue, blood | N/A | N/A | |
| CREATE | 2012 | A variety of tumors with ALK and/or MET alterations | IHC, FISH | Tissue, blood | Central & local | Central | |
| DRUP | 2016 | Advanced or metastatic solid tumor, multiple myeloma or B-cell non-Hodgkin lymphoma | WGS | Tissue, blood | Central | Central | |
| w/a known molecular variant but w/o standard-treatment options | |||||||
| NCI-MATCH | 2015 | Solid tumor, lymphoma, multiple myeloma | NGS | Tissue | Central & local | N/A | |
| NCI-MPACT | 2013 | Advanced solid malignant neoplasm | NGS | Tissue | Central | N/A | |
| OLAPCO | 2015 | DDR-deficient solid tumors | NGS | Tissue, blood | Local | N/A | |
| SHIVA | 2012 | Recurrent or metastatic solid tumors | NGS, IHC, Cytoscan HD | Tissue, blood | Central | Central | |
| TAPUR | 2016 | Solid tumors, multiple myeloma, or B-cell non-Hodgkin lymphoma (pts > 12 years old) | NGS (incl. liquid biopsy), | Tissue, blood | Central | Central | |
| SIT | ARROW | 2017 | Advanced RET-positive solid tumors | NGS, FISH | Tissue, blood | Local | N/A |
| NanoString | |||||||
| CodeBreaK 100 | 2018 | KRAS G12C-mutant NSCLC, colorectal and other solid tumors | NGS, IHC | Tissue, blood | Central | N/A | |
| JAVELIN PARP Medley | 2017 | Locally advanced (primary or recurrent) or metastatic solid tumors | NGS, IHC | Tissue, blood | Central & local | N/A | |
| KEYNOTE-051 | 2015 | Advanced melanoma or PD-L1 positive advanced, relapsed or refractory solid tumor or lymphoma | IHC | Tissue | Central | N/A | |
| LIBTRETTO-001 | 2017 | Advanced RET-positive solid tumors | NGS, FISH, PCR | Tissue, blood | Local | Central (Sponsor) | |
| MyPathway | 2014 | Advanced solid tumors with mutations or gene expression abnormalities predictive of response to trastuzumab/pertuzumab, erlotinib, vemurafenib/cobimetinib, vismodegib, alectinib, or atezolizumab | NGS, WGS, WES, RNA sequencing, FISH, IHC, RT-PCR | Tissue | Local | N/A | |
| NAVIGATE | 2015 | NTRK fusion–positive solid tumors | NGS, FISH | Tissue, blood | Central & local | N/A | |
| ROAR | 2014 | BRAF V600E–mutant rare cancers | IHC, PCR, Sanger sequencing | Tissue, blood | Central & local | N/A | |
| STARTRK-2 | 2015 | Solid tumors that harbor an NTRK1/2/3, ROS1, or ALK gene fusion | NGS | Tissue | Local | N/A | |
| SUMMIT | 2013 | HER (EGFR, HER2) mutation–positive solid tumors | NGS, WES | Tissue | Local | N/A | |
| VE-BASKET | 2012 | BRAF V600 mutation–positive cancers (excluding melanoma and papillary thyroid cancer) | NGS, PCR, Sanger sequencing othersa) | Tissue | Central & local | Central (Sponsor) | |
| Umbrella trial | |||||||
| IIT | FOCUS4 | 2014 | Metastatic colorectal cancer | NGS, IHC | Tissue, blood | Central | Central |
| FUTURE | 2018 | Refractory TNBC who had progressed after standard treatments | NGS, IHC | Tissue | Central | N/A | |
| Lung-MAP | 2014 | sqNSCLC | NGS, IHC | Tissue, blood | Central | N/A | |
| National Lung Matrix Trial | 2015 | NSCLC | NGS | Tissue | Central | N/A | |
| plasmaMATCH | 2016 | Advanced or relapsed breast cancer | NGS, PCR | Blood, tissue | Central | N/A | |
| SUKSES | 2016 | SCLC | NGS, IHC NanoString CNV, FISH | Tissue | N/A | N/A | |
| SIT | BFAST | 2017 | Advanced or metastatic (stage IIIB or IV) NSCLC harboring actionable somatic mutations detected in blood | NGS | Blood | Central | N/A |
| Platform trial | |||||||
| IIT | AcSé-eSMART | 2016 | Relapsed or refractory tumor in pediatric pts (< 18 years old) | WES, NGS (liquid biopsy), whole RNA sequencing | Tissue, blood | Local | Central |
| I-SPY2 | 2010 | Operable, stage II or III breast cancer in neoadjuvant setting | IHC +/– FISH | Tissue | Central | N/A | |
ALK, anaplastic lymphoma kinase; CNV, copy number variation; DDR, DNA damage response and repair; DRUP, Drug Rediscovery Protocol; EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor; IHC, immunohistochemistry; IIT, investigator initiated trials; MATCH, Molecular Analysis for Therapy Choice; MPACT, Molecular Profiling-based Assignment of Cancer Therapy for Patients with Advanced Solid Tumor; MPNST, malignant peripheral nerve sheath tumor; N/A, not available; NCI, National Cancer Institute; NGS, next-generation sequencing; NSCLC, non–small cell lung cancer; PCR, polymerase chain reaction; PD-L1, programmed death-ligand 1; pts, patients; RT-PCR, reverse transcription polymerase chain reaction; SCLC, small cell lung cancer; SIT, sponsor-initiated trials; sqNSCLC, squamous cell non–small cell lung cancer; TAPUR; Targeted Agent and Profiling Utilization Registry; TNBC, triple negative breast cancer; WES, whole exome sequencing; WGS, whole genome sequencing.
3. Independent central review committee, study endpoints, and evaluation criteria
Table 4.
| Study title | ICRC | Primary endpoint | Secondary endpoint | Evaluation criteria | Efficacy outcomes | Met primary endpoint? | FDA approval | Expansion cohort or phase 3 study |
|---|---|---|---|---|---|---|---|---|
| Basket trial | ||||||||
| Ado-Trastuzumab Emtansine basket trial | N | ORR | PFS, toxicity | RECIST | 44% (cohort 1) | Y | N | N |
| ADVL1522 | Na) | ORR, toxicity | PK, CD56 | RECIST | 0-6.25% (stratum 2) | N | N | N |
| ARROW | Y | ORR, toxicity | CBR, DoR, DCR, PFS, OS | RECIST, RANO | 57-71% | Y | Y | Y |
| CodeBreaK 100 | Y | ORR | DoR, DCR, TTR, PFS, OS, Safety | RECIST | 9.7-37.1% | Y (NSCLC) | Y (NSCLC) | Y (NSCLC) |
| CREATE | Y | ORR | Safety, PFS, OS, DCR, DoR | RECIST | 2.5-66.7% | Y (IMFT) | N | N |
| DRUP | N | CBR, toxicity | PFS, OS, DoT | RECIST, IMWG, Lugano, RANO, GCIG | 34% | Y | N | N |
| JAVELIN PARP Medley | Y | ORR | Safety, biochemical response | RECIST, PCWG | 11.1-63.6% | Y (B1, B2, C2) | N | N |
| KEYNOTE-051 | Y | ORR | DoR, PFS, DCR, OS | RECIST, irRECIST, IWG, INRC | 5.9-60% | Y (rrcHL) | Y | N |
| LIBRETTO-001 | Y | ORR | PCSD, DoR, CNS ORR, CNS DoR, TTR, CBR, PFS, OS, Aes | RECIST, RANO | 43.9-85% | Y (1, 2, 3, 4, 7) | Y | N |
| MyPathway | Y | ORR | DCR, PFS, DoR, % of alive participants | RECIST | 23-60% | Y (1) | N | N |
| NCI-MATCH | Y | ORR | OS, PFS | RECIST | 0-38% | Y (H, Z1F) | Y (H) | N |
| NCI-MPACT | N | ORR | PFS rate at 4 mo | RECIST | 0-3.7% | N | N | N |
| NAVIGATE | Y | ORR | DoR, CBR, PFS, OS, AEs, concordance coefficient b/w prior molecular profiling and sponsor’s diagnostic testing | RECIST, RANO | 30-92% | Y | Y | N |
| OLAPCO | N | ORR | PFS, DoR, toxicity | RECIST | 6.7-8.3% | N | N | N |
| ROAR | N | ORR | DoR, PFS, OS, AEs | RECIST, RANO, NCCN, IMWG | 0-89% | Y (1, 2, 4, 7, 8, 9) | Y | Y |
| SHIVA | N | PFS | ORR, toxicity | RECIST | HR 0.88 | N | N | N |
| STARTRK-2 | Y | ORR | DoR, TTP, CBR, intracranial tumor response, CNS PFS, PFS, OS, PK, AE, QoL, bone biomarker | RECIST | 57-67.1% | Y | Y | N |
| SUMMIT | Y | ORR | PFS, ORR, CBR, DoR, OS, AE | RECIST | 0-39% | Y (breast cancer) | N | N |
| TAPUR | Y | ORR, DCRb) | OS, PFS, DoR, toxicity | RECIST | ORR 0-58% | N | N | N |
| DCR 0-69% | ||||||||
| VE-BASKET | N | ORRc) | CBR, ORRc), DoR, TTR, TTP, PFS, OS, safety | RECIST | 7.4-61.5%d) | Y (ECD) | Y (ECD) | Y (ECD) |
| Umbrella trial | ||||||||
| BFAST | Y | ORRe), PFS, DoR | ORRe), DoR, PFS, OS, safety | RECIST | 87.4% (A), 4.5 vs. 4.3 mo (C) | Y (A) | N | N |
| FOCUS4 | Y | PFS | Safety, toxicity, ORR, QoL | RECIST | 3.61-3.88 mo (HR 0.35-0.4) | Y (C, N) | N | N |
| FUTURE | N | ORR | DCR, PFS, OS, safety | RECIST | 0-100% | Y (C, E) | N | N |
| Lung-MAP | Y | ORR | PFS, toxicity | RECIST | 4-16% | Y (A) | N | N |
| National Lung Matrix Trial | N | ORR, DCB rate, PFS | PCSD, TTP, OS, AE | RECIST | PFS 1.9-44.6 mo, DCB rate 7-85%, ORR 3-76% | Y (E, G) | N | N |
| plasmaMATCH | N | ORR | DoR, CBR, PFS, safety/tolerability, mutation frequency, ctDNA accuracy | RECIST | 8-25% | Y (B, C) | N | N |
| SUKSES | N | ORR | DoR, PFS, OS, DCR at 8 wk, AEs, ECG | RECIST | 0% | N | N | N |
| Platform trial | ||||||||
| AcSé-eSMART | N | ORR | PFS, DoR | RECIST, RANO, INRC | 0% | N | N | N |
| I-SPY2 | Nf) | pCRg) | Predictive, prognostic indices for pCR, RCB, RFS, OS, AEs, MRI volume | RCB calculation formulah), SER breast MRI technique for 2nd endpoint | 30-72% | Y (2, 6, 11) | N | N |
AE, adverse event; CBR, clinical benefit rate; CNS, central nervous system; ctDNA, circulating tumor DNA; DCB, durable clinical benefit; DCR, disease control rate; DoR, duration of response; DoT, duration of treatment; DRUP, Drug Rediscovery Protocol; ECD, Erdheim-Chester Disease; FDA, U.S. Food and Drug Administration; ECG, electrocardiogram; GCIG, Gynecological Cancer Intergroup; HR, hazard ratio; ICRC, independent central review committee; IMFT, inflammatory myofibroblastic tumor; IMWG, international myeloma working group; INRC, international neuroblastoma response criteria; irRECIST, immune-related Response Evaluation Criteria In Solid Tumors; IWG, international working group; MATCH, Molecular Analysis for Therapy Choice; MPACT, Molecular Profiling-based Assignment of Cancer Therapy for Patients with Advanced Solid Tumor; MRI, magnetic resoance imaging; NCCN, National Comprehensive Cancer Network; NCI, National Cancer Institute; NSCLC, non–small cell lung cancer; ORR, objective response rate; OS, overall survival; pCR, pathologic complete response; PCSD, percentage change in sum of target lesion diameters; PCWG, Prostate Cancer Working Group; PFS, progressionfree survival; PK, pharmacokinetics; QoL, quality of life; RANO, Response Assessment in Neuro-oncology; RCB, residual cancer burden; RECIST, Response Evaluation Criteria in Solid Tumors; RFS, recurrence-free survival; rrcHL, relapsed or refractory classic Hodgkin lymphoma; SER, signal enhancement ratio; SUKSES, Small cell lung cancer Umbrella Korea StudiES; TAPUR, Targeted Agent and Profiling Utilization Registry; TTP, time to progression; TTR, time to response.
e) Primary endpoints were investigator assessed ORR (cohort A, B, D, and F), PFS (cohort C and G), and DoR (cohort E). Secondary endpoint ORR was facility assessed,
f) The Study Lead Pathologist made the final determination on any indeterminate or contested results,
4. Efficacy results and FDA approval
Table 5.
| Study title | Study type | Year/approval type and indication | Drug(s) | |
|---|---|---|---|---|
| IIT | NCI-MATCH Subprotocol H | Basket | 2022/Accelerated approval for BRAF V600E mutated solid tumors | Dabrafenib+trametinib |
| SIT | ROAR | Basket | ||
| SIT | VE-BASKET | Basket | 2017/Regular approval for BRAF V600E mutated ECD | Vemurafenib |
| KEYNOTE-051 | Basket | 2017/Accelerated approval for MSI-H/dMMR solid tumors | Pembrolizumab | |
| 2023/Full approval for MSI-H/dMMR solid tumors | ||||
| NAVIGATE | Basket | 2018/Accelerated approval for adult and pediatric patients with solid tumors that have a NTRK gene fusion without a known | Larotrectinib | |
| STARTRK-2 | Basket | 2019/Accelerated approval for NTRK fusion solid tumors and ROS1 positive NSCLC | Entrectinib | |
| ARROW | Basket | 2020/Accelerated approval for RET fusion–positive MTCa) | Praseltinib | |
| 2020/Accelerated approval for RET fusion–positive NSCLC | ||||
| 2023/Regular approval for RET fusion–positive NSCLC | ||||
| LIBTRETTO-001 | Basket | 2020/Accelerated approval for RET fusion–positive NSCLC and MTC | Selpercatinib | |
| 2022/Regular approval for RET fusion–positive NSCLC and MTC | ||||
| 2022/Accelerated approval for RET fusion–positive solid tumors other than NSCLC, MTC | ||||
| CodeBreaK 100 | Basket | 2021/Accelerated approval for KRAS G12C mutated NSCLC | Sotorasib | |
dMMR, deficient mismatch repair; ECD, Erdheim-Chester disease; FDA, Food and Drug Administration; IIT, investigator initiated trials; MATCH, Molecular Analysis for Therapy Choice; MSI-H, microsatellite instability high; MTC, medullary thyroid cancer; NCI, National Cancer Institute; NSCLC, non–small cell lung cancer; NTRK, neurotrophic tyrosine receptor kinase; SIT, sponsor-initiated trials.
a) Accelerated approval of praseltinib for MTC was voluntarily withdrawn by Genetech in 2023, following consultation with the FDA and the determination that the confirmatory phase 3 AcceleRET-MTC trial (NCT04760288) required to convert the agent’s accelerated approval to full approval will no longer be pursued due to feasibility.
5. Safety and QoL
Table 6.
| Study title | Planned safety monitoring | ≥ Gr3 TRAE | New safety signalsa) | HRQoL | PROs |
|---|---|---|---|---|---|
| Basket trial | |||||
| Ado-Trastuzumab Emtansine basket trial | Y | 6% | N | N | N |
| ADVL1522 | Y | 20 Events/treatment cycles (n=203) | N/A | N | N |
| ARROW | Y | 49-69% | N | Y (protocol only)b) | N |
| CodeBreaK 100 | Y | 11-16% | N | Y (protocol only)b) | N |
| CREATE | Y | 23.10% | N/A | N | N |
| DRUP | Y | N/A | N/A | N | N |
| JAVELIN PARP Medley | Y | 57% | N/A | N | N |
| KEYNOTE-051 | Y | 8% | N/A | N | N |
| LIBRETTO-001 | Y | 28-40% | N/A | Y (protocol only)b),c) | Y |
| MyPathway | Y | 8-37%d) | N/A | N | N |
| NAVIGATE | Y | 2-25% | N/A | N | N |
| NCI-MATCH | Y | 16-57% | N/A | N | N |
| NCI-MPACT | Y | 45% | N/A | N | N |
| OLAPCO | Y | 32% | N/A | N | N |
| ROAR | Y | 63% | N | N | N |
| SHIVA | Y | 43%e) | N/A | N | N |
| STARTRK-2 | Y | N/A | N/A | N | N |
| SUMMIT | Y | 8% | N/A | Y (protocol only)f) | N |
| TAPUR | Y | 20-43%e) | N/A | N | N |
| VE-BASKET | Y | 17%d) | N | N | N |
| Umbrella trial | |||||
| BFAST | Y | 18% (cohort C) | N/A | Y (protocol only)b) | Y |
| FOCUS4 | Y | 20% (arm D) | N/A | Y (N cohort)f) | N |
| FUTURE | Y | 22% (arm E) | N/A | N | N |
| Lung-MAP | Y | 39.5% (S1400I) | N/A | N | N |
| National Lung Matrix Trial | Y | 53% (arm E) | N/A | N | N |
| plasmaMATCH | Y | 22% (cohort D) | N/A | N | N |
| SUKSES | Y | 60% (arm N3) | N/A | N | N |
| Platform trial | |||||
| AcSé-eSMART | Y | 73.9% (arm A) | N/A | N | N |
| I-SPY2 | Y | 71.0% (veliparib arm) | N/A | Y (protocol only)b) | N |
DRUP, Drug Rediscovery Protocol; HRQoL, health-related quality of life; MATCH, Molecular Analysis for Therapy Choice; MPACT, Molecular Profiling-based Assignment of Cancer Therapy for Patients with Advanced Solid Tumor; NCI, National Cancer Institute; PRO, patient-reported outcome; TAPUR, Targeted Agent and Profiling Utilization Registry; TRAE, treatment-related adverse event.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download